| Cell-Assisted
Lipotransfer (CAL) for Cosmetic Breast Augmentation
-Supportive Use of Adipose-derived Stem/Stromal
Cells-
Kotaro Yoshimura,
M.D.,1 Katsujiro Sato, M.D.,2 Noriyuki Aoi, M.D.,1
Masakazu Kurita, M.D.,3 Toshitsugu Hirohi, M.D.,4
and Kiyonori Harii, M.D.3
|
Introduction
Autologous fat transplantation is one of the promising treatments
for facial rejuvenation and soft-tissue augmentation due
to the lack of incisional scar and complications associated
with foreign materials. However, certain problems remain,
such as unpredictability and a low rate of graft survival
due to partial necrosis. Many innovations have been reported
in an effort to overcome these problems [1, 2, 4-6, 18]
and reviewed previously [4, 14]. Based on these reports
we tentatively concluded that we could harvest fat with
a 2.5 mm cannula or 18-gauge needle at less than 700 mmHg
vacuum and re-inject it with an 18-gauge needle without
significant adipocyte damage [14].
Lipoinjection can be used for treating facial changes associated
with aging, correcting various kinds of depressed deformities
such as hemifacial microsomia and pectus excavatum., It
has also been used in breast augmentation by a limited number
of plastic surgeons [3], although the use of autologous
fat for breast augmentation has been controversial due to
the lack of consensus on whether it is safe and appropriate
because of microcalcifications that may cause confusion
in the evaluation of mammograms. Recently, autologous fat
injection has been re-evaluated as a potential alternative
to artificial implants for breast augmentation [3, 15, 16,
19]. This re-evaluation may reflect recent advances in autologous
fat transfer and the radiological detection of breast cancer.
To overcome the problems associated with autologous fat
transfer, we have used a novel strategy, called Cell-Assisted
Lipotransfer (CAL) (Fig. 1). Tissue-specific progenitor
cells in the adipose tissue were found to have the capacity
to differentiate into various cell lineages [21]. Thus,
the progenitors are now called “adipose-derived stem/stromal
cells (ASCs)”, and expected to become a valuable tool in
a wide range of cell-based therapies. The therapeutic concept
of CAL was described in our previous report on pre-clinical
studies [9]. We found that aspirated fat has approximately
half the number of ASCs than excised whole fat. The two
main reasons for this relative deficiency are 1) a major
portion of the ASCs are located around large vessels and
left in the donor site after liposuction [9], and 2) a part
of ASCs are released into the fluid portion of liposuction
aspirates [14]. The relative deficiency of ASCs may induce
postoperative long-term atrophy of injected fat, as was
partially confirmed in animal studies [8, 9, 11]. In the
CAL strategy, autologous ASCs are used to enhance angiogenesis,
improve the survival rate of grafts, and reduce post-operative
atrophy. In CAL, half the volume of the aspirated fat is
processed for isolation of the stromal vascular fraction
(SVF) containing ASCs. During the isolation process, the
other half of the aspirated fat is prepared for grafting.
Freshly isolated SVF, which we characterized before [20],
is attached to the aspirated fat with the fat acting as
a living scaffold before transplantation. Finally, the SVF-supplemented
fat is injected to the target sites. Thus, ASC-poor fat
is converted to ASC-rich fat in the preparation process
of the injectable material. In this study, we report on
the preliminary results in patients who underwent CAL for
cosmetic breast augmentation. This is the first report of
clinical use of ASCs for cosmetic purposes.
Materials and Methods
Patients
From 2003 to 2007, we have performed CAL in 70 patients;
in the breast in 60 patients (including 8 patients for
breast reconstruction after mastectomy), the face in 12
patients, and the hip in 1 patient (CAL was performed
at 2 sites in 3 patients). Informed consent was obtained
from all patients. The study protocol conformed to the
guidelines of the 1975 Declaration of Helsinki and was
approved by individual institutional review boards.
In this study, 40 patients who had healthy thoraxes and
breasts underwent CAL for purely cosmetic breast augmentation
(i.e. breast reconstruction for inborn anomaly or after
mastectomy was not included). Nineteen of these 40 patients
were followed for more than 6 months (at the time of this
report) and the maximum follow-up period has been 42 months.
All of the patients were Japanese females with a body-mass
index of 19.1 ± 1.9 (mean ± standard deviation) and the
patient’s ages varied from 20 to 62 years (35.8 ± 9.1).
The mean volume of injected fat was 268.1 ± 47.6 ml on
the left side, and 277.3 ± 39.1 ml. Demographic and surgical
data on the patients are summarized in Table 1.
Surgical Techniques
Before the procedure began, the liposuction site was infiltrated
with saline solution with diluted epinephrine (0.001%).
With the patient under general anesthesia, adipose tissue
was suctioned using a cannula with 2.5-mm inner diameter
and a conventional liposuction machine. . About a half
of the collected liposuction aspirate was used for isolation
of the SVF. The SVF was isolated from both the adipose
portion and the fluid portion of liposuction aspirates
as described previously [20] and the cell processing procedure
took about 90 min. During the processing period, the other
half of lipoaspirates was harvested as graft material.
The adipose portion of liposuction aspirates was either
1) washed several times and placed in an upright position
for obtaining clear separation from fluids and oil (Group
A and B), or 2) centrifuged at 700 g for 3 min without
washing (Group C), and put into a metal jar (500 ml) which
was placed in water with crushed ice. In Groups A and
C, the fresh SVF isolated from both the adipose and fluid
portion was added to the graft material and, after gentle
mixing and waiting for 10-15 min for cell adherence to
the aspirated fat, the cell-supplemented fat was then
put into an injection syringe. In Group B, the freshly
isolated SVF was resuspended in 60ml of saline, and diffusely
injected into the whole breast mounds (30 ml for each
breast) separately, just after conventional lipoinjection.
The patient numbers of Groups A, B, and C were 6, 2, and
32, respectively.
For the injection syringe, a 10 cc LeVeen? inflator (Boston
Scientific Corp., MA) or our original syringe (20 ml)
was used because they are screw-type syringes (with a
threaded plunger) and threaded connections that fit both
the connecting tube and the needle, to allow for precise
control during injection (Fig. 2A). To reduce the time
of the procedure, two syringes were used; while one syringe
was being used for an injection, the other was filled
with the graft material in preparation for the next injection.
An 18-gauge needle (150 mm long) was used for lipoinjection
and inserted subcutaneously at one of 4 points indicated
in Fig. 2B. The operator took care to insert and place
the needle horizontally (parallel to the body), in order
to avoid damaging the pleura and causing a pneumothorax.
The needle was inserted in several layers and directions,
and was continuously and gradually retracted as the plunger
was advanced. This technique was used to obtain a diffuse
distribution of the graft material (Figs. 2B, and 3).
The grafts were placed into the fatty layers on, around,
and under the mammary glands, and also into the pectoralis
muscles.
Results
The transplantation of adipose tissue was successfully
performed in all cases, and the time of the injection
process ranged from 35 to 60 min for both breasts. Subcutaneous
bleeding was occasionally seen on some parts of the breasts,
and resolved in one to two weeks.
Transplanted adipose tissue was gradually absorbed during
the first 2 postoperative months (especially during the
first month), and the breast volume showed a minimal change
thereafter, though skin tension sometimes became looser
after 2 months. Photograph of three representative surgical
sites are shown in Figures 4 to 9. Breast circumference
difference (= chest circumference at the nipple ? chest
circumference at the infra-mammary fold) increased in
all cases, by 4 to 8 cm at 6 months, which corresponds
to 2 to 3 cups sizes of brassiere. The increase in the
circumference seems to correspond to 100-200 ml increase
in the volume of each breast mound, which was partially
confirmed by our preliminary evaluation using a 3-dimesional
quantitative measurement system. Compared to breast augmentation
with implants of the same size, augmentation with CAL
showed a lower height but more natural contour of breasts.
All cases but one (see below) showed natural softness
of the breasts without any palpable nodules at 6 months,
and all patients were satisfied with the resulting texture,
softness, contour and absence of foreign materials despite
the limited size increase possible with autologous tissue.
Cyst formation (< 12 mm) was detected by MRI in 2 patients,
and micro-calcification was detected by mammogram in 2
patients at 24 months. In one of 2 patients in group B,
fibrous breast tissue and fibrosis on the sternum were
observed by CT scan at 6 months, and the breasts were
found to be harder than other cases.
Discussion
A number of modifications of lipoinjection techniques
have been attempted in order to improve the survival rate
of injected fat. From these, it is well accepted that
adipose tissue should be placed as small aliquots [3],
preferably within an area 3 mm in diameter [1]. Since
it takes a long time to perform ideally diffuse distribution
of suctioned fat [3], we have used a disposable syringe
with a threaded plunger and connections, a very long needle
(150 mm), and an assistant to rotate the plunger, leading
to only 35-60 min for injection in both breasts. These
devices are critical to performing large-volume lipoinjection
safely and precisely in a short time.
In addition, harvesting, preserving, and refining graft
materials are also important, as repeatedly indicated
in the literature. We used a relatively large-sized suction
cannula, centrifuged the aspirated fat in some cases,
and kept it cooled until transplantation. In this study,
clinical results (increase in breast circumference) appeared
to be superior in Group C using centrifuged fat to Group
A using non-centrifuged fat, though quantitative measurement
and statistical comparison were not done. In a previous
study, we found that centrifugation of aspirated fat is
substantially influential because centrifugation at 1,200
g decreases the fat volume by 30%, damages 12% of the
adipocytes and 0% of the ASCs, which leads to the concentration
of cell numbers per volume of adipocytes and ASCs by 25%
and 43%, respectively [7]. In addition, centrifugation
may be especially beneficial in our treatment, because
water content in the graft material may disturb the adherence
of ASCs to the adipose tissue and interfere with differentiation
into expected lineages. ASCs floating in a solution, which
is a non-physiological environment, may migrate over distances,
penetrate into the lymphatic flow, and differentiate unexpectedly.
We believe that such migration and altered cell-differentiation
caused the development of fibrotic tissue on the sternum
of one patient in Group B. Thus, we conclude that centrifuged
fat combined with ASCs as cell pellets (i.e. Group C)
was best among the three methods used in this study.
Although small cystic formation and micro-calcification
were detected in some cases, the micro-calcification was
easily distinguished from that associated with breast
cancer and the overall cosmetic results were generally
satisfactory and encouraging. Almost all patients were
satisfied with their enlarged and soft breasts with a
natural contour. CT scans and MRI showed that transplanted
fat tissue survived and formed a significant thickness
of the fatty layer not only subcutaneously on and around
the mammary glands but also between the mammary glands
and the pectoralis muscles. Breast volume stabilized 2
to 3 months after transplantation. Maximum breast augmentation
with this technique varied among patients and appeared
to be 100-200 ml. While these volumes may be smaller than
those achieved with large artificial implants, a definite
advantage is that patients do not have to be concerned
about postoperative complications induced by artificial
implants such as rupture, infection, capsular contracture,
unnatural contour, hardness, neurological symptoms and
immune response. Compared to our dozens of patients who
underwent conventional autologous lipoinjection to the
breasts, augmentation effects were apparently higher in
CAL; a 2-3 cm increase in breast circumference was common
in the conventional procedure, but 4-8 cm increase was
seen in this trial of CAL, though the augmentation effect
varied among patients. The measurement system we recently
devised may help to quantify the difference in augmented
volume in the future.
It has been revealed that adipose tissue contains not
only adipogenic progenitor cells but multipotent stem
cells which can differentiate into fat, bone, cartilage,
and types of tissue [21, 22]. Suctioned fat appears to
lose a significant number of these precursors during liposuction
and the preparation processes as compared to non-suctioned
adipose tissue [9]. This relative deficiency of precursors
may contribute to the low survival rate and long-term
atrophy of transplanted lipoaspirates. In CAL, the deficit
of ASCs was compensated for by supplementing ASCs. In
order to maximize the biological function and avoid unexpected
behavior of ASCs, it seems important to ensure adherence
of supplemented ASCs to adipocytes or connective tissue.
There are four possible roles for ASCs in this novel treatment,
which were partly confirmed in pre-clinical studies [8,
9, 11]. First, ASCs can differentiate into adipocytes
and contribute to regeneration of adipose tissue. Second,
ASCs can differentiate into endothelial cells and also
probably into vascular mural cells [8, 10,12], resulting
in the promotion of angiogenesis and graft survival. Third,
ASCs are known to release angiogenic growth factors in
response to hypoxia and other conditions [13], and these
factors influence surrounding host tissue. The last role,
which may be the most influential, is that ASCs survive
as original ASCs [9]. In the adipose, ASCs reside between
adipocytes or in the extracellular matrix, especially
around vessels, and contribute to the turnover of adipose
tissue, which is known to be very slow (2 years or more)
[17]. However, adipose grafts probably turn over during
the first 2 to 3 months after transplantation, because
they experience temporary ischemia followed by reperfusion
injury. This turnover, the replacement process of the
adipose tissue, will be conducted by tissue-specific progenitor
cells, which are ASCs. The relative deficiency of ASCs
in aspirated fat may affect the replacement process and
lead to post-operative atrophy of grafted fat, which is
known to commonly occur during the first 6 months after
lipoinjection.
The freshly isolated SVF used in CAL contains not only
ASCs but also vascular endothelial cells, pericytes, blood
cells (WBCs and RBCs), and other cells as previously described
[20]. ASCs may interact with other cells after transplantation,
such as vascular endothelial cells, and supplementation
with the SVF may be superior to ASCs alone in this treatment.
However, further studies are needed to elucidate the synergistic
effects of ASCs with other cells contained in the graft.
In this preliminary study, satisfactory clinical results
were generally achieved without any major complications.
Thus we can conclude that CAL is safe enough to continue
the study though controlled studies and the accumulation
of long-term results are needed to elucidate the overall
safety and efficacy of the treatment. A variety of new
innovations including stem cell technology may be developed
and contribute to the improvement of autologous tissue
transplantation and regeneration. Further improvements
of the technique may cause autologous tissue transfer
to become the first choice for breast augmentation in
the future.
References
1. Carpaneda CA, Ribeiro MT (1994) Percentage of graft
viability versus injected volume in adipose autotransplants.
Aesthetic Plast Surg 18: 17-19
2. Coleman SR (2001) Structural fat grafts: the ideal
filler? Clin Plast Surg 28: 111-119
3. Coleman SR, Saboeiro AP (2007) Fat grafting to the
breast revisited: safety and efficacy. Plast Reconstr
Surg 119: 775-785
4. Ersek RA, Chang P, Salisbury MA (1998) Lipo layering
of autologous fat: an improved technique with promising
results. Plast Reconstr Surg 101: 820-826
5. Fagrell D, Enestrom S, Berggren A, Kniola B (1996)
Fat cylinder transplantation: an experimental comparative
study of three different kinds of fat transplants. Plast
Reconstr Surg 98: 90-96
6. Har-Shai Y, Lindenbaum ES, Gamliel-Lazarovich A, Beach
D, Hirshowitz B (1999) An integrated approach for increasing
the survival of autologous fat grafts in the treatment
of contour defects. Plast Reconstr Surg 104: 945-954
7. Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K,
Harii K, Yoshimura K. Influences of centrifugation on
cells and tissues in liposuction aspirates: optimized
centrifugation for lipotransfer and cell isolation. Plast
Reconstr Surg, in press.
8. Masuda T, Furue M, Matsuda T (2004) Novel strategy
for soft tissue augmentation based on transplantation
of fragmented omentum and preadipocytes. Tissue Eng 10:
1672-1683
9. Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T,
Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura
K (2006) Cell-assisted lipotransfer: supportive use of
human adipose-derived cells for soft tissue augmentation
with lipoinjection. Tissue Eng 12: 3375-3382
10. Miranville A, Heeschen C, Sengenes C, Curat CA, Busse
R, Bouloumie A (2004) Improvement of postnatal neovascularization
by human adipose tissue-derived stem cells. Circulation
110: 349-355
11. Moseley TA, Zhu M, Hedrick MH (2006) Adipose-derived
stem and progenitor cells as fillers in plastic and reconstructive
surgery. Plast Reconstr Surg 118(3 Suppl):121S-128S
12. Planat-Benard V, Silvestre JS, Cousin B, Andre M,
Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau
C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L
(2004) Plasticity of human adipose lineage cells toward
endothelial cells: physiological and therapeutic perspectives.
Circulation 109: 656-663
13. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove
CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV,
March KL (2004) Secretion of angiogenic and antiapoptotic
factors by human adipose stromal cells. Circulation 109:1292-1298
14. Shiffman MA, Mirrafati S (2001) Fat transfer techniques:
the effect of harvest and transfer methods on adipocyte
viability and review of the literature. Dermatol Surg
27: 819-826
15. Spear SL, Wilson HB, Lockwood MD (2005) Fat injection
to correct contour deformities in the reconstructed breast.
Plast Reconstr Surg 116: 1300-???
16. Spear SL, Newman MK (2007) Discussion to “Fat grafting
to the breast revisited: safety and efficacy”, Plast Reconstr
Surg 119: 786-787
17. Strawford A, Antelo F, Christiansen M, Hellerstein
MK (2004) Adipose tissue triglyceride turnover, de novo
lipogenesis, and cell proliferation in humans measured
with 2H2O. Am J Physiol Endocrinol Metab 286, E577-E588
18. Ullmann Y, Hyams M, Ramon Y, Peled IJ, Leiderbaum
ES (1998) Enhancing the survival of aspirated human fat
injected into nude mice. Plast Reconstr Surg 101: 1940-1944
19. Yoshimura K, Matsumoto D, Gonda K (2005) A clinical
trial of soft tissue augmentation by lipoinjection with
adipose-derived stromal cells (ASCs). Proceedings of the
3rd annual meeting of International Fat Applied Technology
Society (IFATS), pp.9-10, Charlotteville, Virginia.
20. Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki
Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I,
Gonda K (2006) Characterization of freshly isolated and
cultured cells derived from the fatty and fluid portions
of liposuction aspirates. J Cell Physiol 208: 64-76
21. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI,
Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH
(2002) Human adipose tissue is a source of multipotent
stem cells. Mol Biol Cell 13: 4279-4295
22. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz
AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage
cells from human adipose tissue: implications for cell-based
therapies. Tissue Eng 7: 211-228
Legends
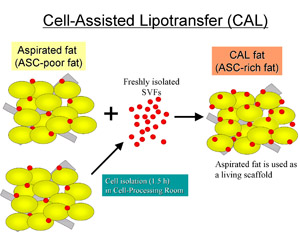
Fig. 1. Scheme of Cell-Assisted Lipotransfer. Relatively
ASC-poor aspirated fat is converted to ASC-rich fat by
supplementing ASCs isolated from the other half of the
aspirated fat. The ASCs are attached to the aspirated
fat, which is used as a scaffold in this strategy.
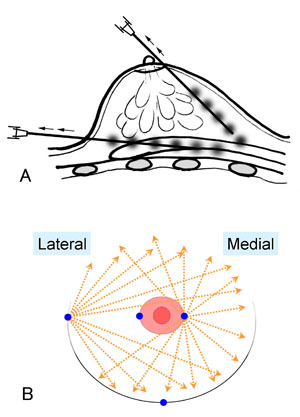
Fig. 2. Schematic instruction of the injection method.
(A) A small amount of fat tissue is injected as small
aliquots or a thin string with a long needle on a syringe
with a threaded plunger while the needle is continuously
withdrawn. (B) The needle is inserted from either one
of two points on the areola margin or one of two points
at the infra-mammary fold in variable directions and planes
to achieve a diffuse distribution.
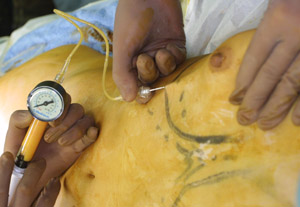
Fig. 3. A clinical view of injection. The injection needle
is rigidly manipulated by an operator, while an assistant
rotates the plunger according to the operator’s instruction.
A high-pressure injection can be performed with a disposable
syringe with a threaded plunger. A 150 mm-long 18-gauge
needle is connected to the syringe with a connecting tube
threaded at both ends.
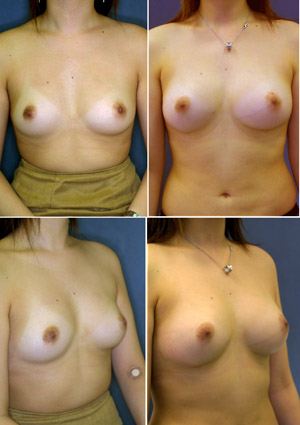
Fig. 4. Clinical views of a patient in Group A (Patient
#1); Preoperative views (top) and postoperative views
at 24 months (bottom). A twenty-two-year-old woman underwent
breast augmentation with CAL (290 ml in each breast) with
satisfactory results at 24 months. Her breast circumference
increased by 5.0 cm. Augmented breast mounds remained
soft and natural appearing without injection scars or
subcutaneous indurations.
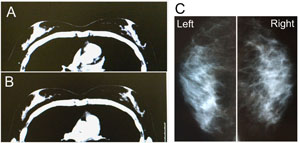
Fig. 5. Radiological views of Patient #1’s chest. (A)
A preoperative CT image in the horizontal plane of the
nipples. (B) A horizontal image 12 months after surgery.
Note that the adipose tissue is augmented both subcutaneously
and under the mammary glands. (C) Mammograms at 12 months
show no calcification or other abnormal signs.
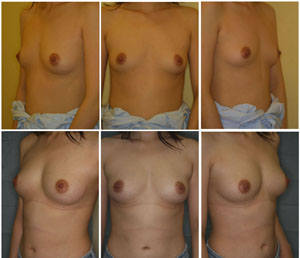
Fig. 6. Clinical views of a patient in Group C (Patient
#2); Preoperative views (top) and postoperative views
at 12 months (bottom). A thirty-two-year-old woman underwent
breast augmentation with CAL (280 ml in each breast).
Her breast circumference difference increased from 9.0
cm (baseline) to 14.5 cm (at 12 months). The breast mounds
are soft and natural appearing with no visible injection
scars.
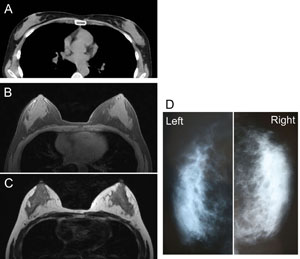
Fig. 7. Radiological views of Patient #2’s chest. (A)
A preoperative CT image in the horizontal plane at the
level of the nipples. (B and C) Horizontal images by MRI
(B, T1-image; C; T2-image) 12 months after surgery. The
adipose tissue is augmented around and under the mammary
glands. A small (< 10 mm) cyst appears in the fatty
layer under the right mammary gland. (D) Mammograms at
12 months show no abnormal signs such as calcifications.
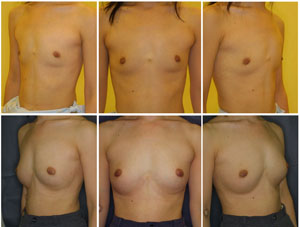
Fig. 8. Clinical views of a patient in Group C (Patient
#3); Preoperative views (top) and postoperative views
at 24 months (bottom). A thirty-old woman underwent breast
augmentation with CAL (310 ml in each breast). Her breasts
were dramatically augmented with an increase in breast
circumference difference by 8.0 cm at 24 months. The breast
mounds were soft with no subcutaneous indurations. An
original infra-mammary fold on the left breast is slightly
visible, but injection scars are not visible.
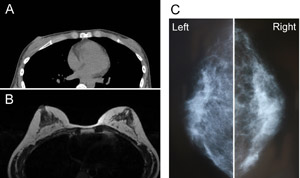
Fig. 9. Radiological views of Patient #3. (A) A preoperative
CT image in the horizontal plane at the level of the nipples.
Only a very thin fatty layer is observed around the mammary
glands. (B) A horizontal MRI image (T1 weighted) 24 months
after surgery. Transplanted adipose tissues survived and
formed thick layers around and under the mammary glands.
(C) Mammograms 24 months after surgery show no abnormal
signs.

