| , INTRODUCTION
One of the requirements for successful cell-based
therapy is the delivery of stem cells to target tissues
after manipulations such as the expansion stem cell
cultures or commitment of stem cells to a specific
lineage. However, due to safety considerations such
as transmission of viral or prion-related disease,
the use of animal-derived products such as serum,
tissue extracts, and enzymes in these manipulations
is undesirable. So, several studies have examined
the use of human-derived substitutes: Attempts were
made to use human autoserum as a replacement for fetal
bovine serum (FBS)1, though its volume available is
limited. Patient-derived fibrin glue (thrombin and
fibrinogen) and platelet-rich plasma have also been
used for cell culture or in clinical trials for enhancing
tissue regeneration2. Other studies have shown that
growth factors derived from platelets can be used
to stimulate cell proliferation3-5, but platelets
can not provide some other major growth factors6.
The growth factor b-FGF, which is an important endogenous
stimulator of angiogenesis7 and cell proliferation8,
is released from surrounding wounded tissues during
an early phase of wound healing9,10. Cellular b-FGF
is released by the lysis of epidermal cells11, fibroblasts11,
and endothelial cells12 around the wound, and b-FGF
bound up in the extracellular matrix is released by
the action of various wound proteases13,14. KGF is
expressed in the dermis during wound healing15, and
stimulates wound reepithelialization16. HGF was independently
discovered as a powerful mitogen for hepatocytes and
as a stimulator of dissociation of epithelial cells17.
HGF-producing cells are found among those of mesenchymal
origin, and HGF stimulates cell proliferation, cell
migration18, and the production of matrix metalloproteinase
(MMP)19 in keratinocytes.
Various growth factors directly or indirectly control
phenomena accompanying wound-healing inflammation,
remodeling and regeneration17, and can be detected
in cutaneous wound fluids20-22, or in wound fluids
obtained through surgical suction drains23-25. The
fluid composition and the concentration of growth
factors in the wound fluids change as healing progresses,
and thus the fluids reflects the sequential wound
healing phenomena. However, there is currently only
limited information pertaining to the characterization
of surgical drainage fluid with regard to growth factors
and other soluble factors. Although a few previous
reports have suggested that wound fluids have mitogenic
and chemotactic effects8,26,27, there has been limited
information beyond that. Also, although it may be
clinically feasible to use wound fluids for cell-based
regenerative therapies, protocols for use of wound
fluids have yet to be optimized.
The present study focuses on the characterization
of subcutaneous wound fluids obtained through surgical
suction drains. Such fluids can be aseptically harvested
with minimal morbidity: for example, adipose-derived
stem cells can be isolated from liposuction aspirates
and subcutaneous wound fluids can be simultaneously
obtained by leaving a suction drainage tube in the
subcutaneous cavity in the same surgery. We sequentially
collected surgical drainage fluids from the subcutaneous
space after plastic surgery, and characterized the
fluids by examining wound healing-associated soluble
factors such as electrolytes, cytokines, chemokines
and MMPs. We compared these characterization profiles
with those of platelet-poor and platelet-rich plasma,
which can be also easily obtained from patients, and
all three types of fluids were assessed for their
potential utility in cell culture.
MATERIALS AND METHODS
Collection and preparation of human
sera from platelet-rich plasma and platelet-poor plasma.
Human platelet-rich plasma (PRP) and platelet-poor
plasma (PPP) were prepared from four healthy volunteers.
Blood was drawn into two 200 ml blood bags containing
0.327% citric acid, 2.63% sodium citrate, 0.0275%
adenine, 0.251% sodium dihydrogenphosphate and 2.9%
D-glucose solution (blood bag CPD-adenineR, Terumo,
Tokyo, Japan). To isolate PRP, bags were centrifuged
at 200 g for 10 min in a large-capacity refrigerated
centrifuge (KUBOTA 9810, KUBOTA Co., Tokyo, Japan),
and to isolate PPP the bags were centrifuged at 5000
g for 5 min; in both instances the supernatant was
harvested. To obtain serum from PRP (designated SPRP)
and from PPP (designated SPPP), 100 ml of PRP or PPP
was drawn into a flask and 200 U of thrombin was added.
The flask was agitated for 60 min at 37C and then
incubated overnight at 4 C, after which the liquid
component was drawn into 50 ml tube, centrifuged at
2000 g for 10 min, and supernatants were obtained
as for SPRP and SPPP. The serum samples were frozen
at -80C and thawed at 37C before analysis.
Collection and preparation of suction
drainage fluid samples
We collected drainage fluids and punctured fluids
from subcutaneous wounds from 15 patients, who underwent
liposuction and abdominoplasty (7 patients), liposuction
(5 patients), and breast augmentation (3 patients)
at the University of Tokyo Hospital. Before samples
were obtained, all patients gave signed informed consent,
as approved by the ethical committee of University
of Tokyo School of Medicine. Two to three weeks before
surgery, 400 ml of blood was harvested from 10 of
the 15 patients. The blood was separated by centrifugation
into concentrated red blood cells and PPP; the cells
were stored in preparation for autotransfusion, and
the PPP was processed to obtain SPPP, using the previously
described method. Suction drains (J-vac drainage system,
Johnson & Johnson, Cornelia, GA, USA) were subcutaneously
inserted into operative wounds during surgery, after
which wound fluid was continuously suctioned at 40-60
mmHg and aseptically collected in the storage bag
of the suction system. The wound fluids were centrifuged
at 2,000 g for 30 minutes, and supernatant fluids
were frozen at -80C. The frozen samples were thawed
at 37C before analysis. Drainage fluid samples (N=52)
obtained from 12 patients whose wounds exhibited normal
healing (NH group), and punctured fluids (n=4) obtained
from 3 patients who had subcutaneous seroma formation
(SF group) were used in this study. Nineteen samples
harvested on day 0 or day 1 from 12 patients of NH
group were referred to as Drainage Fluid-Early (DF-E),
while 9 samples harvested on day 5 or day 6 from 7
of the 12 patients of NH group were referred to as
Drainage Fluid-Late (DF-L). Punctured fluid samples
were harvested by puncturing the subcutaneous seroma
on day 14 or later, and were included in our analyses
as Punctured fluids (PF).
Biochemical analysis of serum and
drainage fluid constituents
From each of 3 patients, we obtained a set of a preoperative
serum samples and 7 sequential drainage fluids (day
0 to day 6). These samples (a total of 8 from each
person) underwent biochemical analyses for total protein,
albumin, sodium, potassium, chloride, calcium, and
iron. Analysis was performed by SRL, Inc. (Tachikawa,
Japan), a commercial analysis service.
Quantitative assays for cytokines,
chemokines, and matrix metalloproteinases associated
with wound healing
Concentrations of various cytokine growth factors
(PDGF-BB, EGF, TGF-ΐ1, b-FGF, VEGF, HGF, KGF and IGF-1)
and chemokines (IL-6 and IL-8) in drainage fluid samples,
punctured fluids, PRP, and PPP were assayed using
anti-human ELISA kits (QuantikineR, R&D Systems
Inc., Minneapolis, MN), according to the manufacturerfs
instructions. Levels of immunoreactive cytokines as
reported by the ELISA assay were measured at 450 nm
by a microplate reader (Model 550, Biorad Laboratories,
Hercules, CA), and a standard curve was generated
to determine growth factor concentrations (pg/mL).
Levels of MMP-1, MMP-8 and MMP-13 in drainage fluids
were also measured using anti-human ELISA kits (Biotrak
ELISA System, Amersham Biosciences, Piscataway, NJ).
Primary cell culture
Adipose-derived stromal cells (ASCs) were isolated
from human lipoaspirates and cultured as previously
described28. Briefly, the suctioned fat was digested
with 0.075% collagenase in PBS for 30 min with agitation
at 37oC. Mature adipocytes and connective tissues
were separated from pellets by centrifugation (800
g, 10 min). Cell pellets were resuspended in erythrocyte
lysis buffer (155mM NH4Cl, 10mM KHCO3, 0.1mM EDTA),
incubated for 5 min at room temperature, resuspended
again and passed through a 100-Κm mesh filter (Millipore,
MA, USA), and then plated at a density of 5 x 106
nucleated cells/100 mm plastic dish. Cells were cultured
in M-199 medium containing 10% FBS at 37oC under 5%
CO2 in a humidified incubator.
Human dermal fibroblasts were isolated from normal
skin samples obtained from plastic surgery. The skin
samples were cut into pieces of approximately 3 ~
3 mm and treated with 0.25 % trypsin in PBS for 24
hour at 4C. After removal of the epidermis, the connective
tissue fragments were attached to 100 mm plastic dishes
and cultured with DMEM containing 10% FBS. Primary
fibroblasts appeared 4 to 7 days after the initiation
of outgrowth cultures and became confluent after 2
to 3 weeks.
Cell proliferation assay using culture medium containing
drainage fluids
Culture media (M199 for ASCs and DMEM for dermal fibroblasts)
containing FBS and/or drainage fluids (DF-E [early]
or DF-L [late]) was prepared at various concentrations.
The drainage fluid samples were sterilized using a
0.22 Κm filter (MILLEX GV Filter Unit, Millipore)
before use. 5x104 cells were plated in 60mm dishes
containing the prepared medium, and the medium was
changed on the third and fifth days. The cell numbers
were counted on the seventh day using a NucleoCounter
(Chemometec, Allerod, Denmark), and average numbers
were calculated from three different cultures of the
cell types for each condition. The averages were normalized
by calculating a ratio of cell numbers grown in each
condition to cell numbers grown in the standard culture
conditions (5% FBS without drainage fluid).
Statistics
The Kruskal- Wallis H- test with post hoc comparisons
was used to compare concentrations of cytokines, chemokines
and MMP among drainages fluids (DF-E and DF-L), punctured
fluids, and SPPP. The Wilcoxon matched matched-pair
t-test was used for comparison between SPRP and SPPP
obtained from the same 4 volunteers, and also to compare
cell numbers in each culture condition with that of
control. P < 0.05 was considered to be significant.
RESULTS
Comparison of cytokine concentrations
in SPRP and SPPP
Concentrations of various cytokine growth factors
in SPRP and SPPP harvested from four volunteers are
shown in Fig. 1. Concentrations of EGF, PDGF, TGF-ΐ,
VEGF and HGF were significantly higher in SPRP than
in SPPP. The difference between the two serums was
markedly seen in concentrations of EGF and PDGF. IGF-1
was similarly contained in both SPRP and SPPP, while
bFGF and KGF were only minimally detected in both.
PDGF detected in SPPP suggested a small amount of
platelet contamination in SPPP.
Biochemical profiles of drainage
fluids and blood serum
Concentrations of total protein and albumin in drainage
fluids on day 0 were about 50% of those in preoperative
serum, and both total protein and albumin gradually
decreased to about 30% of that found in serum by day
6 (Fig. 2). Concentrations of Na+, K+, Cl-, Ca++,
and Fe++ in drainage fluids were similar to those
in serum and did not significantly change with time.
The concentration of Ca++ in drainage fluids was about
60-70% of that in serum and changed very little from
day 0 to day 6. The concentration of Fe++ in drainage
fluid was extremely variable among different patients
due to variations in an individualfs hemorrhage volume,
but in general was substantially greater than that
in serum, and tended to decrease slightly with time.
Cytokine concentrations in drainage
fluids and SPPP
Daily sequential changes in various cytokine growth
factors in drainage fluids from six (VEGF, KGF) or
seven (all other cytokines) patients are shown in
Fig. 3, and data of DF-E (n=19), DF-L (n=9), PF (n=4),
and SPPP (n=10) from patients are summarized in Fig.
4. Concentrations of b-FGF, EGF, PDGF and TGF-ΐ were
much higher in DF-E than in DF-L or SPPP. SPPP contained
much lower concentrations of VEGF, HGF, and KGF compared
to DF-E, DF-L, and PF. PF contained high levels of
TGF-ΐ, VEGF, HGF, and KGF, while concentrations of
b-FGF, EGF and PDGF were sufficiently low in PF that
they could not be detected.
KGF concentrations peaked around day 3 and then began
to decrease, in contrast to VEGF and HGF concentrations,
which steadily increased with successive postoperative
days. IGF-1 did not change significantly with postoperative
time, and the concentration of IGF-1 in DF-E and DF-L
was significantly lower (by approximately 50%) compared
to SPPP.
Chemokine and MMP concentrations
in drainage fluids, SPRP and SPPP
Daily sequential changes in the IL-6 and IL-8 chemokines
and in MMPs (collagenases) were tracked in drainage
fluids from seven patients, and the summarized data
are shown in Fig. 5 and 6. IL-6 was present at high
concentrations in DF-E and decreased gradually in
successive postoperative days, while IL-8 increased
gradually. DF-E contained twice as much IL-6 as was
found in DF-L, while DF-L contained more IL-8 than
DF-E. Neither IL-6 nor IL-8 was detected in SPPP.
PF contained both chemokines, although there were
not significant differences in PF chemokine levels
vs. chemokine levels in either DF-E or DF-L (Fig.
5).
In drainage fluids, MMP-8 was present in much higher
amounts as compared to MMP-1 and MMP-13 (Fig. 5).
MMP-8 increased in the early phase of wound healing,
peaked on day 2 ? 3, and then gradually decreased
with time.
Cell proliferation assays using culture
medium including the drainage fluids
In medium that had not been supplemented with drainage
fluids, ASCs proliferated in a dose-dependent manner
with regard to the concentration of FBS; the dose-dependent
relationship was valid up to 10% FBS (Fig. 7A). When
ASCs were grown in media containing 5% FBS, cell proliferation
was significantly enhanced by the addition of DF-E
or DF-L at concentrations of ?1%. The increase in
proliferation was dose-dependent with respect to the
concentration of DF-E or DF-L. When 5% FBS and 5%
DF-E were added to the medium, cell count increased
to over five times that of the control (5% FBS alone),
and was twice as high as the cell count for media
containing 10% FBS alone. In medium lacking FBS, the
addition of drainage fluids significantly enhanced
ASC proliferation but were less effective compared
to the same concentrations of FBS. We also examined
dermal fibroblasts, which proliferated in a dose-dependent
manner with respect to FBS up to concentrations of
10% FBS (Fig. 7B). Although the proliferation of dermal
fibroblasts was enhanced by drainage fluids in the
absence or presence (5%) of FBS, enhancement of proliferation
by the addition of drainage fluids was moderate. Judged
from theBased on a comparison between 5% FBS+ 5% DF-E
(or DF-L) and 10% FBS, drainage fluids showed no additionalexhibited
no value as an additive to FBS on in terms of expansion
of dermal fibroblasts.
DISCUSSION
Biochemical analysis of drainage fluids
Results from the analysis of the biochemical composition
of drainage fluids in this study were similar to those
of a previous study analyzing biochemical profiles
of drainage fluids after axillary dissection29. Our
analysis showed that concentrations of Na+, K+, and
Cl- in drainage fluids were similar to those in plasma,
while concentrations of Ca++, total protein and albumin
in drainage fluids were ~60 ? 80% lower than those
in plasma. The concentration of Fe++ in drainage fluid
was generally higher than in plasma but was variable,
(depending on the hemorrhage volume) and decreased
over time.
Extracellular fluid volume, which makes up approximately
20% of body weight, is composed of 5% plasma (or intravascular
fluid) and 15% interstitial fluid. The concentrations
of Na+, K+, and Cl- in interstitial fluid are similar
to those in plasma, while the total protein concentration
of interstitial fluid is less than a third of that
of plasma30. It is therefore likely that our drainage
fluids consisted primarily of interstitial fluid,
with plasma composing the remaining ~20-40% of the
total volume. However, drainage fluids are not simply
a mixture of plasma and interstitial fluids because
unlike these fluids, they also contain several other
types of proteins, including various cytokine growth
factors and chemokines.
Cytokine growth factor profiles in
drainage fluids
The sequential changes in cytokine profiles in drainage
fluids shown in this study clearly reflected the successive
activities of various cells and the sequential phenomena
involved in the wound healing process (Fig. S1). In
the early phase (postoperative days 0-1) of wound
healing, b-FGF, PDGF, EGF, and TGF-ΐ1 were present
at high concentrations, and levels subsequently decreased
acutely in the next stage of wound healing. Since
b-FGF is known to be primarily derived from injured
tissue or from cells infiltrating into wounds at early
stages, tissue-bound b-FGF may be released after injury
by several mechanisms, including cell lysis and cell
injury11,12,31. PDGF, EGF, and TGF-ΐ1 were detected
at higher amounts in SPRP than in DF-E, suggesting
that these growth factors were mainly supplied by
dying, lytic platelets in the early phase of wound
healing.
In the second phase of wound healing (postoperative
days 2-4), KFG concentrations peaked, and those VEGF
and HGF slightly increased. Later, in the third phase,
(days 5-6) VEGF and HGF concentrations gradually increased
to peak levels. Since these growth factors were present
at only very low levels on days 0-1, it is possible
that these increases resulted from their release from
the cells that migrated to the wound site after day
1. KFG, VEGF, and HGF are thought to have roles primarily
in granulation, angiogenesis and epithelialization32-34.
KGF is known to be released mainly from fibroblasts
and T cells35,36, VEGF from keratinocytes and macrophages37,38,
and HGF from mesenchymal cells such as dermal fibroblasts18.
Punctured fluids from subcutaneous seroma contained
higher concentrations of growth factors seen in later
phases such as VEGF, HGF, and KGF, but TGF-ΐ1, which
is seen in the early phase, was also abundant in seroma
fluid. This finding may be based on different sources
of the early-phase growth factors: PDGF and EGF are
mostly derived from platelet, whereas TGF-ΐ1 is supplied
not only from platelets but also from various sources.
The initial production of active TGf-ΐ1 from platelets
serves as a chemoattractant for neutrophils, macrophages,
and fibroblasts, and these cells further enhance TGF-ΐ1
production17.
Chemokine and MMP profiles in drainage
fluids
In our study, IL-6, a major mediator of the host response
to tissue injury39, was present in DF-E at about 7000
pg/mL and gradually decreased afterwards. Cells that
appeared in the wound area in each phase seemed to
be major sources of IL-6; neutrophils were present
in the early phase and macrophages and lymphocytes
were present in later phases. In the case of IL-8,
the concentration gradually increased up to day 6,
so it is probable that the fibroblasts present in
the second wave of cell migration to the wound produced
significant amounts of IL-8, as was previously suggested
in a study of fetal wound healing40.
Pro-inflammatory cytokines/chemokines directly stimulate
the synthesis of the collagen-degrading matrix metalloproteinases
(MMPs) and also inhibit the synthesis of tissue inhibitors
of metalloproteinase in fibroblasts and endothelial
cells41. Fibroblasts appear to be the cellular source
for the majority of MMP-142, while neutrophils seem
to provide most of MMP-843. In subcutaneous drainage
fluids, MMP-1 gradually increased up to day 6, while
MMP-8 peaked on days 2 to 3. At all time points examined,
levels of MMP-8 were statistically significantly higher
than both MMP-1 (50-fold to 200-fold) and MMP-13 (1000-fold
to 10000 fold). The sequential changes that we observed
in MMP-1 and MMP-8 were similar to previously reported
data from a study of cutaneous wound fluids44. Taken
together, these data suggest that MMP-8 functions
as the primary debriding collagenase during the acute
phase of wound healing.
Potential use of wound fluids and
SPRP in culture media
Since our data showed that drainage fluids contained
various growth factors which were not found in SPPP
or SPRP, we tested drainage fluids as a substitute
or supplement for serum in the culturing of ASCs and
dermal fibroblasts. The experiment using ASCs showed
that DF-E is superior to FBS as a 5% additive of the
medium containing 5% FBS, while that using dermal
fibroblasts suggested that drainage fluids may be
used as similarly to serum. Thus, we suggest that
drainage fluids may be used as a supplement or substitute
for serum in culture media, and may be able to support
the growth of cell types other than the two lines
examined in this study.
Recent developments in the clinical use of cultured
cells (such as stem cell therapy or gene delivery)
have necessitated safer preparation and manipulation
of cells, which partly entails avoiding the use of
animal-derived serum, tissues and extracts. In this
respect, autologous serum, cytokines or other soluble
factors could be extremely valuable. For example,
ASCs isolated from liposuction aspirates of a patient
could be cultured using the patientfs own SPRP taken
from blood and/or using drainage fluids taken from
the subcutaneous wound after liposuction. Subcutaneous
wound fluids have some advantages compared to cutaneous
and intraperitoneal wound fluids: cutaneous wound
fluids are difficult to collect aseptically and in
a large volume, and intraperitoneal ones can be obtained
only through major abdominal surgery and are not aseptic
in most cases.
The present results could be used as a guide in choosing
the appropriate fluid supplement for cell culture,
based on the specific needs of a given cell line (Table.
S1). Both SPRP and drainage fluids are economical
ready-made mixtures of serum (plasma) and soluble
factors such as cytokines, and can also be used as
raw materials for the extraction of individual soluble
factor proteins. Further investigation will be necessary
to provide optimized protocols for the usage of drainage
fluids and SPRP in cell culture and in factor isolation.
References
1. McAlinden MG, Wilson DJ. Comparison
of cancellous bone-derived cell proliferation in autologous
human and fetal bovine serum. Cell Transplant 2000;
9: 445-51.
2. Thor A, Wannfors K, Sennerby L,
Rasmusson L. Reconstruction of the severely resorbed
maxilla with autogenous bone, platelet-rich plasma,
and implants: 1-year results of a controlled prospective
5-year study. Clin Implant Dent Relat Res 2005; 7:
209-20.
3. Ross R, Glomset J, Kariya B, Harker
L. A platelet-dependent serum factor that stimulates
the proliferation of arterial smooth muscle cells
in vitro. Proc Natl Acad Sci USA 1974; 71: 1207-10.
4. Lucarelli E, Beccheroni A, Donati
D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C,
Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci
P. Platelet-derived growth factors enhance proliferation
of human stromal stem cells. Biomaterials 2003; 24:
3095-100.
5. Eppley BL, Woodell JE, Higgins
J. Platelet quantification and growth factor analysis
from platelet-rich plasma: implications for wound
healing. Plast Reconstr Surg 2004; 114: 1502-8.
6. Sanchez AR, Sheridan PJ, Kupp
LI. Is platelet-rich plasma the perfect enhancement
factor? A current review. Int J Oral Maxillofac Implants
2003; 18: 93-103.
7. Montesano R, Vassalli JD, Baird
A, Guillemin R, Orci L. Basic fibroblast growth factor
induces angiogenesis in vitro. Proc Natl Acad Sci
USA 1986; 83: 7297-301.
8. Nissen NN, Polverini PJ, Gamelli
RL, DiPietro LA. Basic fibroblast growth factor mediates
angiogenic activity in early surgical wounds. Surgery
1996; 119: 457-65.
9. Cordon-Cardo C, Vlodavsky I, Haimovitz-Friedman
A, Hicklin D, Fuks Z. Expression of basic fibroblast
growth factor in normal human tissues. Lab Invest
1990; 63: 832-40.
10. Schulze-Osthoff K, Risau W, Vollmer
E, Sorg C. In situ detection of basic fibroblast growth
factor by highly specific antibodies. Am J Pathol
1990; 137: 85-92.
11. Takimiya M, Saigusa K, Aoki Y.
Immunohistochemical study of basic fibroblast growth
factor and vascular endothelial growth factor expression
for age determination of cutaneous wound. Am J Forensic
Med Pathol 2002; 23: 264-7.
12. Muthukrishnan L, Warder E, McNeil
PL. Basic fibroblast growth factor is efficiently
released from a cytolsolic storage site through plasma
membrane disruptions of endothelial cells. J Cell
Physiol 1991; 148: 1-16.
13. Bashkin P, Doctrow S, Klagsbrun
M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast
growth factor binds to subendothelial extracellular
matrix and is released by heparitinase and heparin-like
molecules. Biochemistry 1989; 28: 1737-43.
14. Ishai-Michaeli R, Eldor A, Vlodavsky
I. Heparanase activity expressed by platelets, neutrophils,
and lymphoma cells releases active fibroblast growth
factor from extracellular matrix. Cell Regul 1990;
1: 833-42.
15. Werner S, Peters KG, Longaker
MT, Fuller-Pace F, Banda MJ, Williams LT. Large induction
of keratinocyte growth factor expression in the dermis
during wound healing. Proc Natl Acad Sci USA 1992;
89: 6896-900.
16. Werner S, Smola H, Liao X, Longaker
MT, Krieg T, Hofschneider PH, Williams LT. The function
of KGF in morphogenesis of epithelium and reepithelialization
of wounds. Science 1994; 266: 819-22.
17. Werner S, Grose R. Regulation
of wound healing by growth factors and cytokines.
Physiol. Rev 2003; 83: 835-70.
18. Matsumoto K, Hashimot K, Yoshikawa
K, Nakamura T. Marked stimulation of growth and motility
of human keratinocytes by hepatocyte growth factor.
Exp Cell Res 1991; 196: 114-20.
19. Dunsmore SE, Rubin JS, Kovacs
SO, Chedid M, Parks WC, Welgus HG. Mechanisms of hepatocyte
growth factor stimulation of keratinocyte metalloproteinase
production. J Biol Chem 1996; 271: 24576-82.
20. Grayson LS, Hansbrough JF, Zapata-Sirvent
RL, Dore CA, Morgan JL, Nicolson MA. Quantitation
of cytokine levels in skin graft donor site wound
fluid. Burns 1993; 19: 401-5.
21. Ono I, Gunji H, Zhang JZ, Maruyama
K, Kaneko F. Studies on cytokines related to wound
healing in donor site wound fluid. J Dermatol Sci
1995; 10: 241-5.
22. Vogt PM, Lehnhardt M, Wagner
D, Jansen V, Krieg M, Steinau HU. Determination of
endogenous growth factors in human wound fluid: temporal
presence and profiles of secretion. Plast Reconstr
Surg 1998; 102: 117-23.
23. Matsuoka J, Grotendorst GR. Two
peptides related to platelet-derived growth factor
are present in human wound fluid. Proc Natl Acad Sci
USA 1989; 86: 4416-20.
24. Baker EA, Gaddal SE, Aitken DG,
Leaper DJ. Growth factor profiles in intraperitoneal
drainage fluid following colorectal surgery: relationship
to wound healing and surgery. Wound Repair Regen 2003;
11: 261-7.
25. Karayiannakis AJ, Zbar A, Polychronidis
A, Simopoulos C. Serum and drainage fluid vascular
endothelial growth factor levels in early surgical
wounds. Eur Surg Res 2003; 35: 492-6.
26. Chen WY, Rogers AA, Lydon MJ.
Characterization of biologic properties of wound fluid
collected during early stages of wound healing. J
Invest Dermatol 1992; 99: 559-64.
27. Trengove NJ, Bielefeldt-Ohmann
H, Stacey MC. Mitogenic activity and cytokine levels
in non-healing and healing chronic leg ulcers. Wound
Repair Regen 2000; 8: 13-25.
28. Yoshimura K, Shigeura T, Matsumoto
D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue
K, Nagase T, Koshima I, Gonda K. Characterization
of freshly isolated and cultured cells derived from
the fatty and fluid portions of liposuction aspirates.
J Cell Physiol 2006; 208: 64-76.
29. Bonnema J, Ligtenstein DA, Wiggers
T, van Geel AN. The composition of serous fluid after
axillary dissection. Eur J Surg 1999; 165: 9-13.
30. Wait RB, Kim UK, Mustafa IA.
Fluids, electrolytes, and acid-base balance. In: Greenfield
LJ, editor. Surgery (third edidtion). Philadelphia;
Lippincott Williams & Wilkins, 2001: 244-69.
31. Baker EA, Leaper DJ. Proteinases,
their inhibitors, and cytokine profiles in acute wound
fluid. Wound Repair Regen 2000: 8: 392-8.
32. Peters KG, De Vries C, Williams
LT. Vascular endothelial growth factor receptor expression
during embryogenesis and tissue repair suggests a
role in endothelial ifferentiation and blood vessel
growth. Proc Natl Acad Sci USA 1993; 90: 8915-9.
33. Gale NW, Yancopoulos GD. Growth
factors acting via endothelial cell-specific receptor
tyrosine kinases: VEGFs, angiopoietins, and ephrins
in vascular development. Genes Dev 1999; 13: 1055-66.
34. Lauer G, Sollberg S, Cole M,
Flamme I, Sturzebecher J, Mann K, Krieg T, Eming SA.
Expression and proteolysis of vascular endothelial
growth factor is increased in chronic wounds. J Invest
Dermatol 2000: 115: 12-8.
35. Brauchle M, Angermeyer K, Hubner
G, Werner S. Large induction of keratinocyte growth
factor expression by serum growth factors and pro-inflammatory
cytokines in cultured fibroblasts. Oncogene 1994;
9: 3199-204.
36. Marchese C, Chedid M, Dirsch
OR, Csaky KG, Santanelli F, Latini C, LaRochelle WJ,
Torrisi MR, Aaronson SA. Modulation of keratinocyte
growth factor and its receptor in reepithelializing
human skin. J Exp Med 1995; 182: 1369-76.
37. Brown LF, Yeo KT, Berse B, Yeo
TK, Senger DR, Dvorak HF, van de Water L. Expression
of vascular permeability factor (vascular endothelial
growth factor) by epidermal keratinocytes during wound
healing. J Exp Med 1992; 176: 1375-9.
38. Frank S, Hubner G, Breier G,
Longaker MT, Greenhalgh DG, Werner S. Regulation of
vascular endothelial growth factor expression in cultured
keratinocytes. Implications for normal and impaired
wound healing. J Biol Chem 1995; 270: 12607-13.
39. Sehgal PB. Interleukin-6: molecular
pathophysiology. J Invest Dermatol 1990; 94 (6 Suppl):
2S-6S.
40. Liechty KW, Crombleholme TM,
Cass DL, Martin B, Adzick NS. Diminished interleukin-8
(IL-8) production in the fetal wound healing response.
J Surg Res 77; 80-4.
41. Murphy G, Willenbrock F, Crabbe
T, O'Shea M, Ward R, Atkinson S, O'Connell J, Docherty
A. Regulation of matrix metalloproteinase activity.
Ann N Y Acad Sci 1994; 732: 31-41.
42. Welgus HG, Jeffrey JJ, Eisen
AZ. The collagen substrate specificity of human skin
fibroblast collagenase. J Biol Chem 1981; 256: 9511-5.
43. Hasty KA, Jeffrey JJ, Hibbs MS,
Welgus HG. The collagen substrate specificity of human
neutrophil collagenase. J Biol Chem 1987; 262: 10048-52.
44. Nwomeh BC, Liang HX, Diegelmann
RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases
MMP-1 and MMP-8 in acute open human dermal wounds.
Wound Repair Regen 1998; 6: 127-34.
Figure Legends
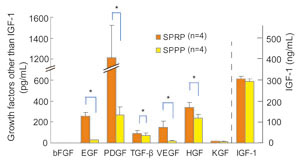
Fig. 1
Comparison of cytokine concentrations in SPRP and
SPPP.
Serums from platelet-rich plasma (SPRP) and from platelet-poor
plasma (SPPP) were collected from four volunteers.
Cytokine concentrations were measured in SPRP and
SPPP. Values represent means } S.E. * P<0.05 (blue
lines), ** P<0.01 (red lines).
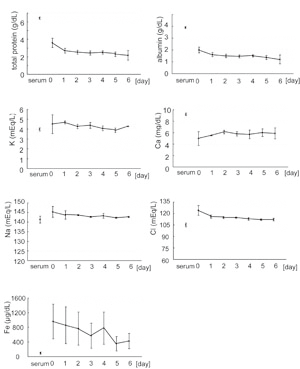
Fig. 2
Biochemical profiles of preoperative serum and of
drainage fluids on days 0 to 6.
Preoperative serum and drainage fluids were collected
from the same three patients; drainage fluids were
collected on days 0-6, where day 0 represents the
day that surgery was performed. The preoperative serum
value is indicated to the left on the x-axis and is
labeled gserumh; the numbers 0-6 to the right represent
day 0 through day 6 timepoints of drainage fluid collection.
Values represent means } S.E.
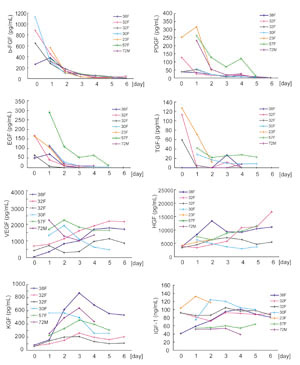
Fig. 3
Daily changes in cytokine concentrations in drainage
fluids.
Drainage fluids were collected on days 0 to 6 from
patients in the normal healing (NH) group, and cytokine
concentrations were examined by ELISA. In some patients,
drainage fluids were not obtained on all the designated
days. For VEGF and KGF, data was derived from six
patients; for all other cytokines, data was derived
from seven patients. Each line shows data from one
patient (e.g. 38F means 38 year-old female).
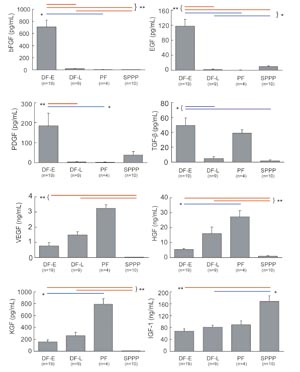
Fig. 4
Comparison of cytokine concentrations in DF-E, DF-L,
PF, and SPPP.
Cytokine concentrations were measured in drainage
fluid samples from day 0 or day 1 (DF-E; Drainage
Fluid-Early), drainage fluid from day 5 or day 6 (DF-L;
Drainage Fluid-Late), punctured seroma fluid samples
from days 14+ (PF; Punctured Fluids), and serum from
platelet-poor plasma (SPPP). Values represent means
} S.E. * P<0.05 (blue lines), ** P<0.01 (red
lines).
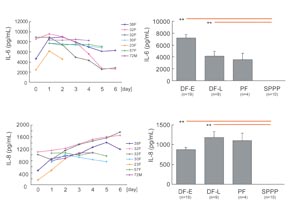
Fig. 5
Changes in IL-6 and IL-8 concentrations in DF-E, DF-L,
PF, and SPPP.
The concentration of IL-6 and IL-8 was measured by
ELISA in drainage fluids collected on days 0-6 and
was similarly measured in PF and SPPP. Data of daily
changes from seven patients were shown in left figures,
in which each line shows data from one patient (e.g.
38F means 38 year-old female). Values in right graphs
represent means } S.E. * P<0.05 (blue lines), **
P<0.01 (red lines).
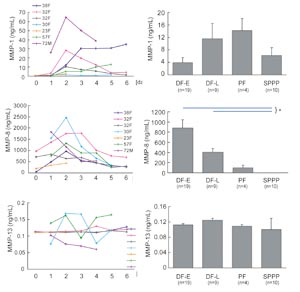
Fig. 6
Changes in concentrations of MMP-1, MMP-8, and MMP-13
in DF-E, DF-L, PF and SPPP.
The concentration of MMP-1, MMP-8, and MMP-13 was
measured by ELISA in drainage fluid collected on days
0-6 and in seroma puncture fluid (PF), SPPP, and SPRP.
Data of daily changes from seven patients were shown
in left figures, in which each line shows data from
one patient (e.g. 38F means 38 year-old female). Values
in right graphs represent means } S.E. * P<0.05
(blue lines).
Fig. 7
Proliferation of ASCs and dermal fibroblasts.
ASCs (A) and dermal fibroblasts (B) were cultured
with DMEM containing various amounts of FBS and/or
drainage fluids (DF-E or DF-L) for 1 week and cell
numbers were counted. Each cell number was expressed
as a ratio to that of the control culture, which was
grown in media that contained 5% FBS and lacked drainage
fluid. Values represent means } S.E. * P<0.05 (bracketed
blue lines), ** P<0.01 (bracketed red lines).
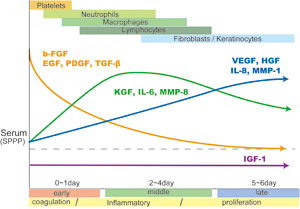
Fig. S1
Summary of sequential changes in
soluble factors associated with wound healing in drainage
fluids from subcutaneous wounds.
There are three types of sequential changes in the
abundance of soluble factors that function in wound
healing. First, levels of b-FGF, EGF, PDGF and TGF-ΐ
are initially high and then gradually decrease. EGF
and PDGF in drainage fluids in the early phase (coagulation
phase) of wound healing are derived from platelets,
although TGF-ΐ is derived from various sources, and
b-FGF is mainly derived from injured tissue or from
cells infiltrating into wounds at early stages. Second,
KGF, IL-6, and MMP-8 peak around days 2 to 4 (during
the inflammatory phase). KGF is released from T lymphocytes
and fibroblasts, while IL-6 seems to be discharged
from the various cells involved in each phase. Third,
VEGF, HGF, IL-8, and MMP-1 are low in the early phase
and gradually increase up to the late phase (proliferation
phase). These factors are derived from cells involved
in the later phases of wound healing, including fibroblasts
and keratinocytes. IGF-1 is present at relatively
consistent levels throughout the entire wound healing
process. MMP-13 is detected only in minimal quantities.
Supplement table
@ drainage fluids serum best source
@ DF-E DF-L SPPP SPRP
@ @ @ @ @ @
bFGF (++) (-) (-) (-) DF-E
EGF (+) (-) (-) (++) SPRP
PDGF (+) (-) (-) (++) SPRP
TGF-ΐ (+) (-) (-) (++) SPRP
VEGF (+) (++) (-) (-) DF-L
HGF (+) (++) (-) (-) DF-L
KGF (+) (++) (-) (-) DF-L
IGF-1 (+) (+) (++) (++) SPPP, SPRP
IL-6 (++) (+) (-) (-) DF-E
IL-8 (+) (++) (-) (-) DF-L
MMP-1 (}) (+) (}) (+) DF-L, SPRP
MMP-8 (++) (+) (-) (-) DF-E
MMP-13 (-) (-) (-) (-) -
@ @ @ @ @ @
Table S1
Sources of autologous soluble factors associated with
wound healing: comparison of drainage fluids, SPPP
and SPRP.
Relative abundances are indicated by -, +/-, +, and
++. ++ indicates high abundance of a factor, and ?
indicates absence of a factor.
|

