|
ABSTRACT
It was recently revealed that epidermal growth following
topical treatment with all-trans retinoic acid (atRA) was
at least partly induced by heparin-binding EGF-like growth
factor (HB-EGF) released from suprabasal keratinocytes. Since
proliferation of keratinocytes appears to be one of the critical
roles of atRA in depigmentation treatment and promotion of
wound healing, HB-EGF is supposed to be suitable for assessing
the therapeutic value of topical retinoids. In this study,
HB-EGF mRNA expression in normal human keratinocytes after
atRA treatment was examined, and the effects of a variety
of natural and synthetic retinoids were compared to each
other.
The present results of RT-PCR suggested that induction of
differentiation increased HB-EGF mRNA expression in cultured
keratinocytes. Real-time PCR analyses revealed that HB-EGF
mRNA expression was elevated dose-dependently with atRA,
most at 12 hours. This elevation was more prominent in keratinocytes
of confluent state than those of subconfluent state, suggesting
differentiated keratinocytes are more subject to be stimulated
by atRA with regard to HB-EGF expression than proliferating
keratinocytes.
HB-EGF mRNA was upregulated in differentiation-induced keratinocytes
by all retinoids used in this study at 1 μM, and marked upregulation
was seen when treated with three isotypes of retinoic acid
(atRA, 9-cis and 13-cis retinoic acid). RARα-selective agonists
(Am80, Am580, ER-38925, and TAC-101) and a panagonist of
RARs (Re80) showed relatively low elevation of HB-EGF transcripts
as well as all-trans retinol (Rol) and all-trans retinal
(Ral). Although another panagonist (Ch55) showed the highest
elevation of HB-EGF mRNA, it was relatively cytotoxic at
the concentration employed. Ral and Rol was found to upregulate
HB-EGF when used at 100 μM to 1 mM, to a similar extent of
atRA at 1 to 10 μM. The capacity of retinoids to upregulate
HB-EGF may be an important index to develop and seek an ideal
synthetic retinoid, which has maximum benefits and minimal
side effects.
INTRODUCTION
Topical tretinoin (all-trans retinoic acid; atRA) has been
widely used for several skin diseases such as acne vulgaris
and photoaged skin with remarkable success since 1970's.
Retinoids have a variety of biological effects on epidermis
and dermis including appendices, and those are mediated by
specific nuclear receptors; RARs (retinoic acid receptors)
and RXRs (retinoid X receptors) (1, 2). Epidermal hyperplasia
is one of the most prominent histological changes in skin
seen after treatment with atRA (1, 3). This phenomenon was
commonly observed not only in vivo in several mammals, but
also in skin equivalents using normal human keratinocytes
(NHK) and fibroblasts (data not shown). However, since NHK
proliferation was not consistently observed in monolayer-cultured
NHK (2, 4), the mechanism of keratinocyte proliferation induced
by retinoids remained unknown for a long time.
Recently, a study using cultured keratinocytes, organ culture
and skin biopsies, revealed that transcripts of heparin-binding
EGF-like growth factor (HB-EGF), a member of the EGF family
of growth factors, are induced by treatment of retinoids,
suggesting that epidermal hyperplasia after atRA treatment
may be mediated at least in part by keratinocyte-derived
HB-EGF (5). HB-EGF was shown to be upregulated in actual
wound healing much more than other growth factors that accelerate
epidermal growth (6). Thereafter, a paracrine action of HB-EGF
released from suprabasal keratinocytes was found to be a
key mechanism of epidermal growth following atRA treatment
by a study using transgenic mice (7). Thus, it is suggested
that atRA accelerates keratinocyte differentiation in a direct
manner, and promotes keratinocyte proliferation in an indirect
manner.
Combination topical therapies with atRA and hydroquine for
pigmented skin lesions have been successfully performed since
Kligman and Willis proposed their depigmenting formula in
1975 (8). The authors modified the protocol and demonstrated
the depigmenting potential of atRA (9, 10). Since atRA appeared
not to have a suppressive effect on melanogenesis, keratinocyte
proliferation and acceleration of epidermal turnover (keratinocytes
differentiation) appear to be the two critical roles of atRA
in the depigmenting therapies (11). Since the former of the
two is mediated by a paracrine action of HB-EGF released
from suprabasal keratinocytes, HB-EGF mRNA is supposed to
be suitable for assessing the therapeutic value of topical
retinoids as far as they are used for treating hyperpigmentation
or promoting wound healing.
The purposes of this study are to reconfirm the effect of
atRA to promote the expression of HB-EGF mRNA in normal human
keratinocytes, and to compare the HB-EGF-promoting abilities
of a variety of natural and synthetic retinoids. The capacity
of retinoids to upregulate HB-EGF may be an important index
to develop and seek an ideal synthetic retinoid, which has
maximum beneficial effects and minimal side effects.
METHODS
Cell isolation and cell culture
Human epidermal keratinocytes isolated from biopsies of healthy
skin obtained from young Japanese patients during plastic
surgery were used in this study. Keratinocytes were isolated
using a modification of the method reported previously (12).
Briefly, the specimens were washed 3 times in phosphate buffered
saline (PBS) and finely shredded with scissors, and incubated
with 0.25 % trypsin and 0.02 % EDTA in PBS for 16-24 hours
at 4 ?C. The epithelium was separated from the dermis with
forceps, and keratinocytes were isolated from the subepithelial
side. Keratinocytes were grown in a modified serum-free Keratinocyte
growth medium (KGM; Kyokuto Seiyaku, Tokyo), which consists
of MCDB153 with high concentrations of amino acids, transferrin
(final concentration 10 μg/ml), insulin (5 μg/ml), hydrocortisone
(0.5 μg/ml), phosphorylethanolamine (14.1 μg/ml), and bovine
pituitary extract (40 μg /ml). The final concentration of
Ca2+ in the medium was 0.03 mM.
Keratinocytes in subconfluent state (30 % confluency) or
those in confluent state (cultured for 48 hours after they
showed 100 % confluency) were used in this study, representing
growing keratinocytes and differentiated ones, respectively.
Reagents
The influences of various kinds of retinoids on HB-EGF mRNA
expression of keratinocytes were compared in this study.
Five kinds of natural retinoids and 6 kinds of synthetic
retinoids were used (Fig. 1). For natural retinoids,
tretinoin (all-trans retinoic acid; atRA), 13-cis retinoic
acid (13cRA), 9-cis retinoic acid (9cRA), all-trans retinol
(Rol), and all-trans retinal (Ral) were used. All of
the natural retinoids were purchased from Sigma (St Louis,
MO). 9cRA is known to be a ligand for RXRs and bind also
to RARs. For synthetic retinoids, AM80 (4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)-carbamoyl]
benzoic acid), Am580 (4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carboxamido]
benzoic acid), Ch55 ((E)-4-[3-(3,5-di-tert-butylphenyl)-3-oxo-1-propenyl]
benzoic acid), and Re80 (4-[1-hydroxy-3-oxo-3-(5,6,7,8-tetrahydro-3-hydroxy-5,5,8,8-tetramethyl-2-
naphthalenyl)-1-propenyl] benzoic acid) were generous gifts
from Dr. Kagechika (University of Tokyo, Tokyo), TAC101 (4-[3,5-bis(trimethylsilyl)
benzamide] benzoic acid) and ER-38925 (4-[5-(4,7-dimethylbenzofuran-2-yl)pyrrol-2-yl]
benzoic acid) were also generous gifts from Taiho Parmaceuticals
Co, Ltd (Tokyo, Japan) and Eisai Co, Ltd. (Tokyo, Japan),
respectively. Am80, Am580, TAC-101, and ER-38925 are RARα-selective
agonists (13-16), while Ch55 and Re80 are panagonists for
all three RAR subtypes (12, 17). All reagents were dissolved
in ethanol at 1 mM as stock solutions (for atRA, Rol, and
Ral; other stock solutions were also prepared), and 10 μl
of each stock solution was added to 10 ml of the culture
medium to get the designated final concentrations (for comparison
of all retinoids; 1 μM). As a control, 10 μl of ethanol alone
was added to 10 ml of the culture medium.
RNA isolation
After removing the culture media and washing twice with PBS,
total RNA was obtained with RNeasyR Mini Kit (QIAGEN,
Hilden, Germany). In order to eliminate residual genomic
DNA, RNase-Free DNase Set (QIAGEN, Hilden, Germany) was
also used after. The concentration of each RNA sample
was measured with Spectrophotometer V-530 UV/VIS (JASCO,
Tokyo, Japan).
Reverse transcription-PCR
(RT-PCR) analysis
The amount of isolated total RNA was spectrophotomecally
measured. A reverse transcriptase reaction was performed
using RNA PCR Kit (AMV) Ver.2.1 (TaKaRa, Tokyo, Japan) according
to the instruction manual; 5 μg of total RNA in a 100 μM
of reaction mixture (final concentrations: 5 mM MgCl2, 1
mM dNTP Mixture, 1 U/μl RNase Inhibitor, 0.125 μM Oligo dT-Adaptor
Primer, 10 mM Tris-HCl, 50 mM KCl, pH 8.3) containing 25
units of AMV Reverse Transcriptase XL at 42?C for 30 minutes,
followed by inactivation of the enzyme at 99 ?C for 5 minutes
with Program Temp Control System PC-700 (ASTEC, Fukuoka,
Japan). The control reaction was performed simultaneously
under identical conditions, but without reverse transcriptase.
For PCR amplification, 0.5 μl of each cDNA reaction mixture
was added to 49.5 μl PCR mixture containing 5 μl MgCl2 (25
mM), 5 μl 10X PCR buffer, 1 μl deoxynucleotide mixture (10
mM), 0.5 μl each of the 3' and 5' primers (50 μM each), and
0.25 μl Taq polymerase, and 37.25 μl DEPC-treated water.
Reaction mixtures were amplified using Microplate Gradient
Thermal Cycler PC-960G (Corbett Research, Australia). The
PCR cycle conditions were: melting for 30 seconds at 94 ?C,
annealing for 30 seconds at 59 ?C, and extension for 1.5
minutes at 72 ?C. The oligonucleotide primers used for RT-PCR
were as follows; human HB-EGF (forward) 5'- CACACCAAACAAGGAGGAGC
-3' and (reverse) 5'- CATGAGAAGCCCCACGATGA -3' (PCR-product
size: 279bp); human glyceraldehydes-3-phosphate dehydrogenase
(GAPDH), (forward) 5'- GAAGGTGAAGGTCGGAGTC -3' and (reverse)
5'- GAAGATGGTGATGGGATTTC -3' (PCR-product size: 226bp). All
reverse transcription-PCR products were separated on 2 %
agarose gels, visualized by ultraviolet B using ethidium
bromide staining.
Real time RT-PCT analysis
Expression of HB-EGF transcripts by keratinocytes was quantitatively
measured using real-time quantitative PCR System (Sequence
Detection System ABI PRISM 7700, PE Applied Biosystems,
Foster City, CA). Real-time PCR was performed on ABI
PRISMTM 96-Well Optical Reaction Plates (PE Biosystems,
Foster City, CA). All PCR reaction mixtures per well
contained 25 μl of TaqMan SYBRR Green PCR Master Mix
(PE Biosystems, Foster City, CA), 0.25 μl of forward
primer (10 pmol/μl), 0.25 μl of reverse primer (10 pmol/μl),
4 μl of each diluted sample (RT products), 20.5 μl of
distilled water. PCR amplification of each sample was
performed with both specific primer pairs of target human
HB-EGF gene and human GAPDH gene on the same reaction
plate. The oligonucleotide primers used for real-time
PCR were as follows; human HB-EGF (forward) 5'- CAGATCTGGACCTTTTGAGAGTCA
-3' and (reverse) 5'- TCCCGTGCTCCTCCTTGTT -3' (PCR-product
size: 78bp); human GAPDH, (forward) 5'- GAAGGTGAAGGTCGGAGTC
-3' and (reverse) 5'- GAAGATGGTGATGGGATTTC -3' (PCR-product
size: 226bp). The PCR reaction was comprised of 40 cycles,
consisting of denaturing at 95 ?C (15 sec.), annealing/extension
at 60 ?C (1 min.). For comparison of the HB-EGF mRNA
expression, the value of HB-EGF of each sample was normalized
by deduction by the value of GAPDH of the same sample.
In order to eliminate the possibility of contamination
of genomic DNA during extraction of total RNA, a control
reaction with each primer pair was performed at the same
time under identical condtions without reverse transcription,
and no amplification was detected. The normalized data
were collected by at least 4 separate experiments.
Statistical analysis
In each experiment, the values of normalized HB-EGF mRNA
expression of each sample were devided by the value of
the control to obtain data for analysis. Statistical
analysis was performed using Student t-test.
RESULTS
I. RT-PCR analysis
In preliminary experiments, HB-EGF mRNA expression was examined
with RT-PCR. HB-EGF mRNA expression was elevated in keratinocytes,
differentiation-induced by incubation for 48 hours with high
concentration (1.8mM) of Ca2+ or 2% serum, or both, relative
to the control (Fig. 2). This finding suggested that normal
human keratinocytes increase HB-EGF mRNA expression when
they differentiate.
The investigation of sequential changes of HB-EGF mRNA elevation
by atRA (1 μM) revealed that HB-EGF mRNA expression increased
most at 12 hours after atRA administration (Fig. 3).
In addition, our results also suggest that HB-EGF transcripts
were dose-dependently elevated by incubation with atRA for
15 hours in the concentration range of 0.01 μM to 1 μM (Fig.
4).
II. HB-EGF mRNA elevation
by atRA in keratinocytes in subconfluent or confluent
condition
The sequential changes of HB-EGF mRNA elevation by atRA (1
μM) was quantitatively measured with real-time PCR system
using normal human keratinocytes in subconfluent (30% confluence)
and confluent (100% confluence) condition (Fig. 5). For the
confluent condition, keratinocytes cultured for 48 hours
after they were first observed confluent in culture dishes
were used, representing differentiated keratinocytes as a
model of suprabasal keratinocytes. For the subconfluent condition,
keratinocytes showing 30% confluency were used immediately,
representing proliferating keratinocytes as a model of basal
keratinocytes. The normalized HB-EGF mRNA level was highest
at 12 hours, and thereafter decreased in both cases. Throughout
the time investigated (0-48 hours), the average value of
HB-EGF mRNA level was higher in the confluent condition than
the subconfluent condition. Statistical significance between
the subconfluent condition and the confluent one was found
only at 12 hours.
III. Comparison of HB-EGF
mRNA upregulation by various kinds of natural and
synthetic retinoids
The HB-EGF mRNA levels were quantitatively measured with
real-time PCR in normal human confluent-state keratinocytes
cultured for 15 hours with 11 kinds of retinoids (0.01 μM,
0.1 μM, and 1 μM for atRA; 1 μM for the other retinoids).
The results demonstrated that all of the 11 kinds of retinoids
studied showed significant upregulation of HB-EGF mRNA, yet
to different extents (Fig. 6). Three isotypes of retinoic
acid (atRA, 9cRA, and 13cRA) showed higher degrees of upregulation
of HB-EGF transcripts than any other natural or synthetic
retinoids except Ch55, although there was no apparent difference
among the three retinoic acids. Ch55, a panagonist of RARs,
demonstrated a much higher degree of upregulation of HB-EGF
transcripts than the three retinoic acids, but the amount
of total RNA isolated from keratinocytes treated with Ch55
was very small compared to that from the other samples, suggesting
Ch55 was cytotoxic to keratinocytes in the concentration
employed here (1 μM). RARα-selective synthetic agonists (Am80,
Am580, ER-38925, and TAC-101) and another panagonist of RARs
(Re80) showed a lesser degree of upregulation of HB-EGF transcripts
than the three retinoic acids, as well as the other two natural
retinoids, Rol and Ral.
IV. Comparison of upregulations
of HB-EGF mRNA by atRA, Rol, and Ral.
HB-EGF transcripts expression of normal human confluent-state
keratinocytes treated for 15 hours with several concentrations
of atRA, Rol and Ral were quantitatively measured using real-time
PCR. Compared at 1 μM and 10 μM, HB-EGF was upregulated significantly
more by atRA than by Rol or Ral (Fig. 7). When using the
concentration of 100 μM to 1 mM, atRA was cytotoxic, whereas
Rol and Ral markedly upregulated HB-EGF to a similar degree
to atRA at 1 μM to 10 μM. This means that even Rol and Ral
could promote keratinocyte proliferation as well as atRA
when used at higher concentrations (presumably 40-100 fold).
It remained unknown whether Ral and Rol upregulated HB-EGF
through nuclear receptors directly or after oxidative conversion
to atRA. An alternative pathway can not be fully excluded.
DISCUSSION
It was recently revealed that HB-EGF mRNA was induced by
treatment of retinoids in human keratinocytes and organ-cultured
skin, suggesting that epidermal hyperplasia after atRA treatment
may be mediated at least in part by keratinocyte-derived
HB-EGF(5). Thereafter, a paracrine action of HB-EGF released
from suprabasal keratinocytes was suggested to be a key mechanism
of epidermal growth following atRA treatment by the study,
in which dominant-negative RARα was overexpressed in suprabasal
layers of mice and the response of skin to atRA was examined
(7). The present study confirmed that HB-EGF mRNA was markedly
induced by atRA in human normal keratinocytes, and suggested
that even cell differentiation of keratinocytes alone might
increase keratinocyte-derived HB-EGF without retinoid treatment.
Although the previous study (5) did not detect a significant
difference in HB-EGF upregulation between confluent and subconfluent
keratinocytes at 6, 24, 48 hours, our results revealed that
differentiated keratinocytes in confluent condition upregulated
HB-EGF significantly more than growing keratinocytes in subconfluent
condition after 12 hours of atRA treatment, supporting the
hypothesis of Xiao et al. (7) that HB-EGF released from differentiated
suprabasal keratinocytes stimulate proliferation of basal
keratinocytes. Whether the induction of HB-EGF mRNA is due
to ligand-dependent transcription activation of the HB-EGF
gene or not is to be explored, because the presence of retinoic
acid response elements (RARE) has not yet been identified
in the promoter region of the HB-EGF gene (5, 7).
As far as depigmenting treatment is concerned, promotion
of keratinocyte proliferation and acceleration of kerationcyte
differentiation appeared to be the two main roles of atRA
(11). Assumed that HB-EGF released from suprabasal keratinocytes
is the main reason for epidermal growth following retinoid
treatment in vivo, the promotive ability of retinoids on
HB-EGF expression can be used as an index in evaluating individual
retinoid and developing the synthetic retinoids to promote
epidermal growth. However, any reliable index indicating
the ability of retinoids to differentiate keratinocytes has
not been found.
All natural and synthetic retinoids employed in this study
significantly upregulated HB-EGF, suggesting that epidermal
growth can be induced by a wide range of topical retinoids.
However, the extent of HB-EGF upregulation was distinct among
those agents. All four RARα-specific synthetic reinoids,
Am80, Am580, ER-38925, and TAC-101, showed relatively low
promotion of HB-EGF mRNA to all three isotypes of retinoic
acids, whereas those synthetic retinoids demonstrated a higher
affinity to RARα and higher activity than atRA in other actions
such as growth suppression of neoplasm (13-16). The reason
for the variance in promotion of HB-EGF mRNA among retinoids
remains unknown, but it may be partly due to differential
binding affinity to RARγ. Ninety percent of RARs expressed
in epidermis is composed of RARγ, and the other of RARα,
while RXRα is a major RXRs in skin (2, 18). Both RARs and
RXRs were reported to be expressed much higher in the spinous
and granular layers in comparison to the basal layer in normal
skin (19). Since RAR-β, or RAR-γ specific ligands were not
evaluated in this study, the relationship between retinoid
specificity to subtypes of RARs and HB-EGF expression requires
further investigation.
In addition to mediating RARE- or RXRE(retinoid X receptor
responsive elements)- dependent transactivation, retinoid
receptors can also affect gene expression by inhibiting the
action of other transactivation factors, including AP-1 (20).
Furthermore, there are some RAR/RXR-independent pathways,
such as activation of the mannose-6-phosphate (M6P)/insulin-like
growth factor-II (IGF-II) (21).
Human keratinocytes are known to transform Rol into Ral,
and then into atRA by two enzymatic steps involving dehydrogenases,
and the conversion rate of Rol into atRA depends on the state
of keratinocytes differentiation; differentiated keratinocytes
can convert at a higher rate than non-differentiated keratinocytes
(22). The binding affinity of Rol or Ral to retinoid nuclear
receptors is quite low (23), so that their biological activity
should result from their oxidative transformation into retinoic
acid by epidermal keratinocytes. However, there have been
some reports suggesting the existence of other pathways than
that mediated by nuclear receptors (24).
Rol is constitutively present in human plasma at 1-2 μM (25)
and the upper limit of extracellular Rol concentration in
epidermis is thought to be 0.7 μM (26), whereas atRA is present
at 4-14 nM in human plasma (27, 28). The present results
revealed that all of atRA, Rol and Ral induce markedly HB-EGF
mRNA at pharmacological concentration which is 100-1000 folds
higher than physiological concentration. Even Rol and Ral
induced high level of HB-EGF mRNA when used at much higher
(40-100 fold) concentration than atRA. This means that Rol
and Ral when used at high concentration could promote epidermal
growth in vivo as well as atRA, although Rol and Ral have
been reported to show much less side effects than atRA when
used at the similar concentration. Rol and Ral are though
to be more tolerable because of less side effects, and may
be of great value in clinical use.
The results indicated the potentiality of use of Rol or Ral
at high concentration in vivo, especially in depigmenting
treatment which needs promotion of epidermal growth and turnover.
Indeed, our preliminary clinical study with 5-10% Rol aqueous
gel suggested similar depigmenting effects to 0.1% atRA aqueous
gel (in preparation). Thus, promotion of HB-EGF expression
may be used as one of promising indexes to evaluate the depigmenting
ability of topical retinoids.
ACKNOWLEDGEMENTS
We thank Dr. Hiroyuki Kagechika (University of Tokyo, Tokyo,
Japan) for his generous provision of Am80, Am 580, Ch55,
and Re80. We also acknowledge Yuka Kuwahara for her technical
assistance.
REFERENCES
1) Kligman A M, Grove G L, Hirose R, Leyden J J. Topical
tretinoin for photoaged skin. J Am Acad Dermatol 1986: 15:
836-859.
2) Fisher G J, Voorhees
J J. Molecular mechanisms of retinoid actions in
skin. FASEB J 1996:10: 1002-1013.
3) Tur E, Hohl D, Jetten
A, Panizzon R, Frenk E. Modification of late epidermal
differentiation in photoaged skin treated with topical
retinoic acid cream. Dermatology 1995:191: 124-128.
4) Gibson D F, Bikle
D D, Harris J. All-trans retinoic acid blocks the
antiproliferative prodifferentiating actions of 1,25-dihydroxyvitamin
D3 in normal human keratinocytes. J Cell Physiol
1998: 174: 1-8.
5) Stoll S W, Elder J
T. Retinoid regulation of heparin-binding EGF-like
growth factor gene expression in human keratinocytes
and skin. Exp Dermatol 1998: 7: 391-397.
6) Stoll S, Garner W, Elder J. Heparin-binding ligands mediate
autocrine epidermal growth factor receptor activation In
skin organ culture. J Clin Invest 1997: 100:1271-1281.
7) Xiao J H, Feng X,
Di W, et al. Identification of heparin-binding EGF-like
growth factor as a target in intercellular regulation
of epidermal basal cell growth by suprabasal retinoic
acid receptors. EMBO J 1999: 18:1539-1548.
8) Kligman A M, Willis
I. A new formula for depigmenting human skin.
Arch Dermatol 1975: 111: 40-48.
9) Yoshimura K, Harii
K, Aoyama T, Shibuya F, Iga T. A new bleaching protocol
for hyperpigmented skin lesions with a high concentration
of all-trans retinoic acid aqueous gel. Aesthetic
Plast Surg 1999: 23: 285-291.
10) Yoshimura K, Harii
K, Aoyama T, Iga T. Experience with a strong bleaching
treatment for skin hyperpigmentation in Orientals.
Plast Reconstr Surg 2000: 105:1097-1108.
11) Yoshimura K, Tsukamoto
K, Okazaki M, et al. Effects of all-trans retinoic
acid on melanogenesis in pigmented skin equivalents
and monolayer culture of melanocytes. J Dermatol
Sci 2001: 27: S68-75.
12)Tsunenaga M, Kohno
Y, Horii I, et al. Growth and differentiation properties
of normal and transformed keratinocytes in organotypic
culture. Jpn J Cancer Res 1994: 85: 238-244.
13) Hashimoto Y, Kagechika
H, Kawachi E, Shudo K. Specific uptake of retinoids
into human promyelocytic leukemia cells HL-60 by
retinoid-specific binding protein: possibly the true
retinoid receptor. Jpn J Cancer Res 1988: 79: 473-483.
14) Kagechika H, Kawachi
E, Hashimoto Y, Himi T, Shudo K. Retinobenzoic acids.
1. Structure-activity relationships of aromatic amides
with retinoidal activity. J Med Chem 1988: 31: 2182-2192.
15) Murakami K, Wierzba
K, Sano M, Shibata J, Yonekura K, Hashimoto A, Sato
K, Yamada Y. TAC-101, a benzoic acid derivative,
inhibits liver metastasis of human gastrointestinal
cancer and prolongs the life-span. Clin Exp Metastasis
1998: 16: 323-331.
16) Yoshimura H, Kikuchi
K, Hibi S, et al. Discovery of novel and potent retinoic
acid receptor alpha agonists: syntheses and evaluation
of benzofuranyl-pyrrole and benzothiophenyl-pyrrole
derivatives. J Med Chem 2000: 43: 2929-2937.
17) Kagechika H, Kawachi
E, Hashimoto Y, Shudo K. Retinobenzoic acids. 2.
Structure-activity relationships of chalcone-4-carboxylic
acids and flavone-4'-carboxylic acids. J Med Chem
1989: 32: 834-840.
18) Fisher G J, Talwar H S, Xiao J H, et al. Immunological
identification and functional quantitation of retinoic acid
and retinoid X receptor proteins in human skin. J Biol Chem
1994: 269: 20629-20635.
19) Xu X C, Lotan R.
Aberrant expression and function of retinloid receptors
in cancer. In: Nau H, Blaner W S, ed. Retinoids.
Berlin: Springer, 1999: 335-336.
20) Schule R, Rangarajan
P, Yang N, et al. Retinoic acid is a negative regulator
of AP-1-responsive genes. Proc Natl Acad Sci USA:
1991: 88: 6092-6096.
21) Kang JX, Li Y, Leaf
A. Mannose-6-phosphate/insulin-like growth factor-II
receptor is a receptor for retinoic acid. Proc Natl
Acad Sci USA 1997: 94:13671-13676.
22) Siegenthaler G, Saurat
J H, Ponec M. Retinol and retinal metabolism. Relationship
to the state of differentiation of cultured human
keratinocytes.
Biochem J 1990: 268: 371-378.
23) Crettaz M, Baron
A, Siegenthaler G, Hunziker W. Ligand specificities
of recombinant retinoic acid receptors RAR alpha
and RAR beta. Biochem J 1990: 272: 391-397.
24) Saurat J H, Sorg
O, Didierjean L. New concepts for delivery of topical
retinoid activity to human skin. In: Nau H, Blaner
W S, ed. Retinoids. Berlin: Springer, 1999: 521-538.
25) Soprano D, Blaner
W S. Plasma retinol-binding protein. In: Sporn MB,
Roberts AB, Goodman DS, ed. The retinoids: biology,
chemistry, and medicine, 2nd edn. New York: Raven,
1994: 257.
26) Randolph R K, Siegenthaler
G. Vitamin A homeostasis in human epidermis: native
retinoid composition and metabolism. In: Nau H, Blaner
W S, ed. Retinoids. Berlin: Springer, 1999: 499-500.
27) DeLeenHeer A P, Lambert
W E, Claeys I. All-trans retinoic acid: Measurement
of reference values I human serum by high performance
liquid chromatography. J Lipid Res 1982: 23: 1362-1367.
28) Eckhoff C, Nau H.
Identification and quantitation of all-trans- and
13-cis retinoic acid and 13-cis-4-oxo-retinoic acid
in human plasma. J Lipid Res 1990: 31: 1445-1454.
LEGENDS
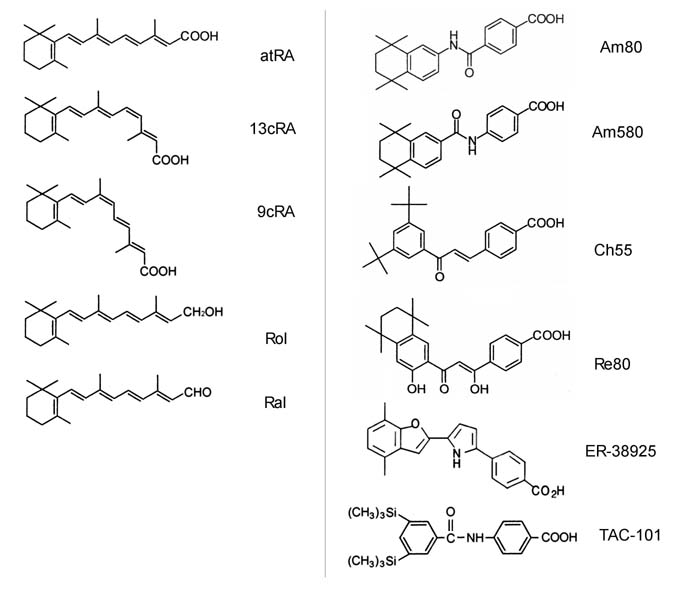
Fig.1. Chemical structures
of retinoids used in this study. (left) 5 kinds of
natural retinoids; atRA: all-trans retinoic acid,
13cRA: 13-cis retinoic acid, 9cRA: 9-cis retinoic
acid, Rol: all-trans retinol, Ral: all-trans retinal.
(Right) 6 kinds of synthetic retinoids.
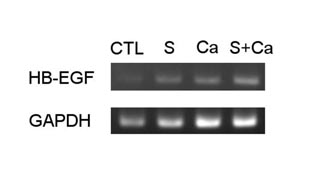
Fig.2. HB-EGF mRNA expression
in keratinocytes differentiation-induced by 48 hours
incubation with the medium containing 2 % serum or/and
1.8 mM Ca2+. It was amplified by RT-PCR and showed
along with control incubated with normal medium.
CTL: control, S: the medium contained 2 % serum,
Ca: the medium contained 1.8mM Ca2+, S+Ca; the medium
contained both 2% serum and 1.8mM Ca2+.
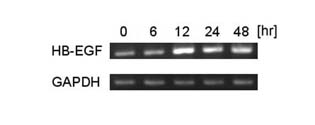
Fig.3. HB-EGF mRNA expression
in keratinocytes cultured with medium containing
1 μM atRA for 0, 6, 12, 24, and 48 hours. The expression
was amplified by RT-PCR.
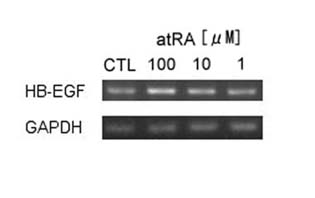
Fig.4. HB-EGF mRNA expression
in keratinocytes cultured with medium containing
3 kinds of concentration (1 μM, 0.1 μM, and 0.01
μM) atRA for 15 hours. The expression was amplified
by RT-PCR.
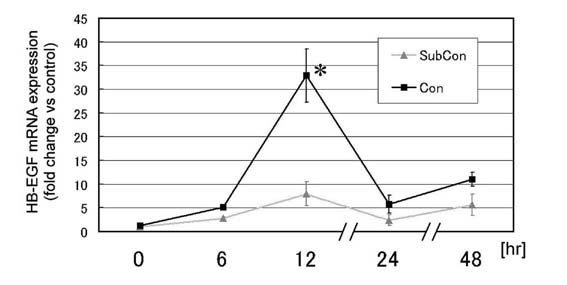
Fig.5. HB-EGF mRNA expression
in keratinocytes in subconfluent (30 %) or confluent
(100 %) condition cultured with medium containing
1 μM atRA for 0, 6, 12, 24, and 48 hours. HB-EGF
mRNA was measured quantitatively with real-time PCR
system and the normalized value was calculated by
deducting by the GAPDH value of each sample. The
values are demonstrated as relative values to the
value obtained from the subconfluent keratinocytes
at 0 hour, and indicated as mean + standard error.
: p<0.05.
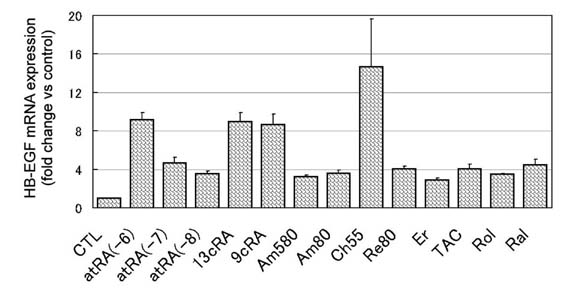
Fig.6. HB-EGF mRNA expression
in normal human keratinocytes in confluent condition
cultured for 15 hours with 1 μM each retinoid (0.01,
0.1, 1μM for atRA). HB-EGF mRNA was measured quantitatively
with real-time PCR system. The values are demonstrated
as relative values to the value obtained from the
control in each measurement, and indicated as mean
+ standard error. CTL: control, atRA(-6): 1 μM atRA,
atRA(-7); 0.1 μM atRA, atRA(-8): 0.01 μM atRA, Er:
ER-38925, TAC: TAC-101. The data of all retinoids
are significantly higher than that of control.
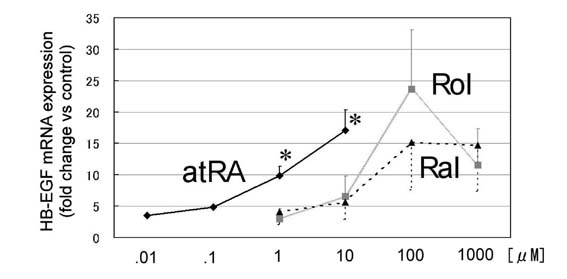
Fig.7. HB-EGF mRNA expression
in normal human keratinocytes in confluent condition
cultured for 15 hours with one of the three natural
retinoids (atRA, Rol, and Ral). HB-EGF mRNA was measured
quantitatively with real-time PCR system. The values
are demonstrated as relative values to the value
obtained from the control (normal medium) in each
measurement. Four kinds of concentration (0.01 μM,
0.1 μM, 1 μM, and 10 μM for atRA; 1 μM, 10 μM, 100
μM, and 1 mM for Rol and Ral) were employed for each
measurement. The values are indicated as mean + or
- standard error. At the concentration of 1 μM and
10 μM, HB-EGF mRNA expression was significantly higher
in keratinocytes treated with atRA than those treated
with Rol or Ral. : p<0.05.
|

