Abstracts
The effects of all-trans retinoic acid (RA) on melanogenesis
and the mechanism of its action in topical treatment
have not been elucidated. The purpose of this study is
to determine the effects of RA on melanogenesis in the
pigmented skin equivalent as well as in monolayer culture
of melanocytes, and to determine whether RA, hydroquinone
(HQ), and hydrocortisone (HC) show synergistic depigmenting
effects in combined treatments of each other.
The suppressing effects of RA on melanogenesis were not observed
in pigmented skin equivalents and monolayer culture of murine
and human melanocytes, although HQ shows strong inhibition
of melanogenesis. The synergistic effects between RA, HQ, and
HC were not particularly seen.
The results suggested that RA neither have direct inhibitory
effects on melanogenesis of melanocytes, nor influence the
cell-cell interactions between melanocytes, keratinocytes and
fibroblasts, such as paracrine actions with regard to melanin
productions. The role of RA in bleaching treatments appears
to be other specific actions, such as promotion of keratinocytes
proliferation and acceleration of epidermal turnover.
Introduction
All-trans retinoic acid (tretinoin; RA) has been clinically
used for acne and photoaged skin for more than two decades.
Topical application of RA is known to be also effective for
melasma and some other skin hyperpigmentation in a single [1-4]
or combined use [5-8] with some other reagents such as hydroquinone
(HQ) and corticosteroids. Since Kligman et al. [5] proposed
a combined use of RA, HQ and dexamethasone as a topical depigmenting
formula, a number of products based on the formula have become
commercially available and widely used for bleaching of hyperpigmented
skin lesions.
Hydroquinone is a most widely used depigmenting agent at present
and has been shown to inhibit tyrosinase-mediated conversion
of tyrosine to dopa [9] and dopa to dopaquinone [10]. Corticosteroids
are also known to have a depigmenting effect in a single use
[11], and this is one of the reasons why Kligman et al. [5]
employed corticosteroid in his formula.
Although it is known that topical RA improves a variety of
skin hyperpigmentation, the mechanism underlying these depigmenting
effects of RA has not been elucidated. Furthermore, although
a number of studies have been performed on effects of RA on
pigment cells, the experimental results remain contradictory
[12]. Some studies with mouse melanoma cell lines and human
melanocytes indicated a pigmentation-promoting effect of RA
[13-16]. On the other hand, there was no melanogenic effect
on K-1735P ultraviolet-irradiated, transformed murine melanoma
cells, which remained amelanotic following RA treatment [17].
Under conditions which supported proliferation, RA at concentrations
of 0.25-0.1Êg/ml inhibited growth of human melanocytes, while
there was no significant change in the amount of melanin per
cell or in tyrosinase activity [18]. It is known that tyrosinase
gene expression and activity, if already stimulated by MSH,
is inhibited by RA in murine and hamster melanoma cells [19],
whereas RA treatment caused a marked increase in MSH binding
capacity for both cell surface and internal MSH binding sites
[20]. Another report indicated that RA significantly decreased
the UVB-stimulated melanogenesis through suppression of tyrosinase
and TRP-1 synthesis at the post-transcriptional level in mouse
melanoma cells and human melanocytes [21]. Thus, it is difficult
to establish an unequivocal effect of RA on melanogenesis in
pigment cells.
Several growth factors have been identified as the paracrine
factors from keratinocytes and fibroblasts, showing effects
on melanocyte proliferation and functions [22-25]. Three-dimensional
pigmented skin equivalent is a useful strategy to investigate
the cell-cell interactions in the regulation of in vivo melanogenesis,
and the effects of RA has not ever been examined with the pigmented
skin equivalent. The purpose of this study is to determine
the effects of RA on melanocytes in monolayer culture and also
in pigmented skin equivalents consisting of melanocytes and
keratinocytes grown on a dermal equivalent, and to determine
whether RA and/or HC show synergistic depigmenting effects
in a combined use with HQ.
Materials and method
Melanocyte culture
The murine melanocyte cell lines, melan-a, and conditions
for its culture have been described previously [26]. Melan-a
was cultured in Dulbecco's modified Eagle's minimum essential
medium (DMEM) supplemented with 5% fetal calf serum (FCS),
100 ÊM -mercaptoethanol, 2mM L-gultamine, and 200nM TPA (tumor
promoter 12-O-tetradecanoylphorbol acetate). Normal human
melanocytes obtained from Asian foreskin and serum-free growth
medium were purchased from Morinaga Institute of Biological
Science (Yokohama, Japan). Human melanocytes were cultured
in MM-4 medium [27] supplemented with 10Êg/l phorbol-12-myristate
13-acetate, 10Êg/l cholera toxin, and 150mg/l bovine pituitary
extract.
RA was obtained from Sigma Chemicals (St. Louis, MO), dissolved
at 10-3M in ethanol, and kept in foil-wrapped containers
protected from light at 4 C. HQ and hydrocortisone (HC) were
obtained from Wako Pure Chemical Industries (Osaka, Japan).
Murine melanocytes (Melan-a) or human melanocytes were seeded
in 3.5 cm dishes (1.5x105 cells/dish) for melanin and protein
assay. Human melanocytes were cultured in MM-4 with 0.5 %
serum for 24 h, after which the medium was exchanged for
MM-4 without serum. Melan-a was cultured in DMEM with TPA
for 2 days, after which the medium was exchanged for DMEM
without TPA. After murine or human melanocytes had been cultured
for 4 days, every assay was performed. RA (10-6M), HQ (10-5M),
and HC (10-4M) were individually or in combination of each
other added to each medium for 4 days, and seven kinds of
culture groups were prepared as follows; none (control),
RA alone (RA), HQ alone (HQ), HC alone (HC), RA+HQ, RA+HC,
HQ+HC, and RA+HQ+HC. At least 4 data were collected for each
group.
Pigmented skin equivalents
Human keratinocytes and fibroblasts were isolated from skin
sections obtained from skin surgery for young patients.
Human keratinocytes were grown in a modified serum-free
KGM (Kyokuto Seiyaku, Tokyo), which consists of MCDB153
with high concentrations of amino acids, transferrin
(final concentration, 10 Êg/ml), insulin (5 Êg/ml), hydrocortisone
(0.5 Êg/ml), phosphorylethanolamine (14.1 Êg/ml) and
bovine pituitary extract (40 Êg/ml). Human fibroblasts
were grown in DMEM supplemented with 10% FCS. Keratinocytes
and fibroblasts at population doubling levels of 2-4
and 10-15, respectively, were used for experiments.
Human keratinocytes and murine melanocytes were cultured
in a three-dimensional fashion at the air-liquid interface
on top of a dermal equivalent consisting of cultured fibroblasts
(106/gel) and type I collagen. The dermal equivalents (contracted
collagen gels) were prepared according to the method described
by Tsunenaga et al. [28]. Murine melanocytes were plated
at 2x105/cm2 inside a glass ring (12 mm diameter) on the
surface of the dermal equivalent, which was then placed on
a stainless steel mesh. Melanocytes were grown in DMEM plus
10% FCS on day 1. On day 2, keratinocytes were additionally
plated at 8x105/cm2 inside the glass ring on the dermal equivalent,
and the medium was changed to 1:1 mixture of KGM and DMEM
plus 10% FCS, in which the Ca2+ concentration was adjusted
to 0.18mM. After day 4, the medium in the glass ring was
removed and the surface of the dermal equivalent on which
keratinocytes and melanocytes were cultured were allowed
to face the air. The discharged medium in the ring was aspired
twice a day after day 4. The medium in the dish was changed
every other day. The medium including the designated compounds
was used since day 4. On day 9, skin equivalents were fixed
with 4% paraformaldehyde in PBS.
Measurement of pigment
on the skin equivalents
The melanin pigments of each sample were photographed and
each digital image was analyzed with Image-Pro Plus (version
3.0, Media Cybernetics, Silver Spring, Maryland). Difference
between an original image and its extracted background was
estimated as the melanin pigments, and area and intensity
of each pigment were measured. Total sum of melanin pigments
data of each sample was referred to as "total melanin" of
individual samples in this paper.
Melanin and protein
assay
Melanin content was determined according to the method described
by Oikawa and Nakayasu [29], which we modified. Briefly,
Melan-a or human melanocytes were cultured at 37 ?C for four
days with the various types of medium. Cell pellets were
lysed in 1.0 ml extraction buffer (50mM Tris buffer; pH7.5,
2mM EDTA, 150mM NaCl, and 1% Triton-X). Supernatants were
used for protein assay, which was performed with BCA protein
assay kit (Pierce, Rockford, IL). After resuspending melanin
pellets in 1.0 ml extraction buffer and centrifuging, the
melanin pellets were incubated with 0.5 ml 2N NaOH containing
melanin 20% DMSO for 30 min. The optical absorbance of each
sample was measured at 470 nm with an ELISA plate reader
(Model 550 microplate reader, Bio-Rad Laboratories, CA).
Tyrosinase assay
Compounds were tested for direct effects on tyrosinase activity
using a modified radiometric tyrosinase assay as previously
described [30]. Briefly, the melanogenic tyrosinase assay
was performed in quadruplicate in 96-well microtiter
plates by adding 10 Êm compound and 20 Êl purified murine
or human tyrosinase, in that order. After 30 min preincubation
at 23 C, 10 Êl of L-[14C] tyrosine was added along with
10 Êl 0.25 mM L-Dopa cofactor in 1 M sodium phosphate
buffer, pH 7.2, containing 0.01% albumin. Reactions were
incubated for 1 h at 37 ?C, after which 100 Êl 0.1 M
HCl with excess unlabelled L-tyrosine was added to each
well. The contents of each well were removed with a multichannel
pipettor to a dot-blot apparatus (Bio-Rad, Hercules,
CA) and acid-insoluble radioactive melanin and melanin
precursors were bound to ZetaProbe blotting membranes
(Pharmacia, Piscataway, NJ) for 15 min at 23 C. The membranes
were then dried under vacuum and washed three times with
250 Êl 0.1 M HCl with excess unlabelled tyrosine; they
were then removed from the apparatus and washed three
more times for 20 min each with 100ml 0.1 M HCl. Membranes
were then air-dried and exposed to a Storm phosphor screen;
quantitation of radioactive melanin production on those
blots was performed using a Strom 860 PhosphorImager
and ImageQuant software (Molecular Dynamics).
Statistics
Significant differences were sought using two way analysis
of variance (ANOVA). Post hoc comparison of individual
group was performed only if the F ratio for the overall
ANOVA was significant; appropriate Bonferonni corrections
were applied to all post hoc comparisons. Differences
were considered significant when p values < 0.05.
Results
Melanin pigments on
pigmented skin equivalents (Figs. 1, 2, 3)
The representative histology of the control sample was shown
in Fig. 1, and representative samples of each group were
demonstrated in Fig. 2. Total melanin was significantly reduced
in HQ-, RA+HQ-, HQ+HC-, and RA+HQ+HC-treated groups (Fig.
3). Total melanin of RA-treated group was elevated by 10%
in average, but the difference was not statistically significant.
Total melanin was significantly less in HQ-, HC-, RA+HQ-,
RA+HC-, HQ+HC-, and RA+HQ+HC-treated groups than in RA-treated
group.
Melanin content and
total protein in murine melanocytes (Fig 4)
Total protein in any group except for RA+HQ+HC-treated group
was not significantly different from that in the control.
Total melanin (absolute value) did not show a large change
after RA-, HC-, or RA+HC-treatment. The relative melanin
in RA-, HC-, and RA+HC-treated groups was elevated by 10-35%
in average value, but the difference was not statistically
significant. The absolute and relative values of melanin
were significantly reduced in HQ-, RA+HQ-, and RA+HQ+HC-treated
groups.
Melanin content and
total protein in human melanocytes (Fig. 5)
Total protein in any group was not significantly different
from that in the control. In RA-treated group, the absolute
and relative melanin was elevated by 20-30% in average value,
nevertheless without statistical significance. The absolute
and relative values of melanin were significantly reduced
in HQ- and RA+HQ-treated groups.
Tyrosinase activity
in murine melanocytes (Fig. 6)
HQ has an obvious inhibition on tyrosinase activity up to
70%. RA and HC has also slight inhibition on tyrosinase activity.
Synergistic effects of three compounds were not seen in this
study.
Tyrosinase activity
in human melanocytes (Fig. 7)
RA appeared to show no suppressing effects. HQ showed 60%
decrease in melanogenic activity. HC also showed decrease
in melanogenic activity, but no synergistic effects in three
compounds.
Discussion
The mechanisms by which RA and HQ act in the combined bleaching
protocols such as in Kligman's regimen and others are
still to be elucidated. Although a number of studies
have been performed concerning the effects of RA on skin
in vivo or in vitro, there are some contradictory results
the reasons for which remain unknown [31-33]. Although
it is reported that even the topical application of RA
alone has clinically a depigmenting effect [1-3], the
suppressive effects of RA on melanocyte growth and melanogenesis
have not been established in vitro [12]. Welsh et al.
[34] reported that topical application of RA to the mice
skin increases the number of activated epidermal melanocytes
and makes melanocytes more sensitive to activation by
ultraviolet B radiation.
In the present study, suppressing effects of RA on melanogenesis
of melanocytes, were not observed both in monolayer culture
and organotypic culture. Melanin production per cell was
not significantly changed in monolayer culture of the murine
melanocyte cell line and human melanocytes, although proliferation
of melanocytes and tyrosinase activity were somewhat suppressed
by RA treatment in monolayer culture of murine melanocytes.
In pigmented organotypic culture consisting of murine melanocytes
and human keratinocytes grown on a dermal equivalent, melanogenesis
was even promoted, although the difference in total melanin
production between control and RA-treated group was not statistically
significant. The results suggested that RA neither have direct
inhibitory effects on melanogenesis of melanocytes, nor influence
the cell-cell direct and indirect interactions between melanocytes,
keratinocytes and fibroblasts in paracrine manner with regard
to melanin production.
In monolayer culture of murine and human melanocytes, all
experimental groups with treatment of HQ suppressed melanin
production and tyrosinase activity. HC did not show apparent
suppressing effects on melanogenesis and tyrosinase activity
in monolayer culture. Neither RA nor HC show synergistic
suppressing effects of HQ on melanogenesis and tyrosinase
activity.
The results of the present study suggested that RA does not
have suppressing effects on melanogenesis of melanocytes
in spite of its clinical whitening efficacy on some pigmented
skin lesions, nor synergistic suppressive effects with HQ.
Therefore, it is suggested that the depigmenting effects
in vivo of RA in a single or combined use with HQ and/or
HC are derived from other mechanisms than direct effects
on melanocytes. A recent report suggested a role for cellular
retinoic acid binding protein (CRABP)-I in mediating RA effects
on melanogenesis and involvement of keratinocytic and dermal
influences in CRABP activity in melanocytes [35]. However,
in our pigmented skin equivalents, in which interaction between
keratinocytes, melanocytes and fibroblasts can be examined,
inhibitory effects of RA on melanogenesis were not observed.
RA promotes not only proliferation but also turnover of keratinocytes
in vivo, and compaction of the horny layer and hyperplasia
of the epidermis are characteristic changes after the topical
application of RA [31]. It is also known that RA can promote
collagenogenesis in dermis and wound healing [36, 37]. On
the other hand, skin becomes atrophic after the application
of corticosteroid [38, 39] and corticosteroid suppresses
collagenogenesis and wound healing [40]. Thus, corticosteroid
appears to be antagonistic in skin to retinoids in some aspects
[36, 37, 39, 41]. In our clinical experiences, depigmenting
effects of a combined treatment of RA and HQ were suppressed
by corticosteroid [7, 8], although corticosteroids are known
to have a depigmenting effect with single use [11]. Taken
together, it is speculated that the promotion of keratinocyte
proliferation and acceleration of keratinocyte differentiation
are most likely to be the roles of RA in the depigmenting
treatment. Melanin granules may be washed out of the epidermis
by the fast and strong stream of keratinocytes in the epidermis
induced by the mechanism above. These effects could not be
reproduced in our pigmented skin equivalents, which can be
cultured for only one week after they were exposed to the
air.
References
1) Rafal ES, Griffiths CEM, Ditre CM, Finkel LJ, Hamilton
TA, Ellis CN, Voorhees JJ. Topical tretinoin (retinoic acid)
treatment for liver spots associated with photodamage. N
Engl J Med 1992;326:368-74.
2) Griffiths CEM, Finkel LT, Ditre CM, Hamilton TA, Ellis
CN, Voorhees JJ. Topical tretinoin (retinoic acid) improves
melasma: a vehicle-controlled, clinical trial. Br. J Dermatol
1993;129:415-21.
3) Griffiths CEM, Goldfarb MT, Finkel LJ, Roulia V, Bonawitz
M, Hamilton TA, Ellis CN, Voorhees JJ. Topical tretinoin
(retinoic acid) treatment of hyperpigmented lesions associated
with photoaging in Chinese and Japanese patients: a vehicle-controlled
trial. J Am Acad Dermatol 1994;30:76-84.
4) Gano SE, Garcia RL. Topical tretinoin, hydroquinone, and
betamethasone valerate in the therapy of melasma. Cutis 1979;23:239-41.
5) Kligman AM, Willis I. A new formula for depigmenting human
skin. Arch Dermatol 1975;111:40-48.
6) Pathak MA, Fitzpatrick TB, Kraus EW. Usefulness of retinoic
acid in the treatment of melasma. J Am Acad Dermatol 1986;15:894-9.
7) Yoshimura K, Harii K, Shibuya F, Aoyama T, Iga T. A new
bleaching protocol for hyperpigmented skin lesions with a
high concentration of all-trans retinoic acid aqueous gel.
Aesthetic Plast Surg 1999;23:285-91.
8) Yoshimura K, Harii K, Aoyama T, Iga T. Experience of a
strong bleaching treatment for skin hyperpigmentation in
Orientals. Plast Reconstr Surg 2000;105:1097-108.
9) Denton CR, Lerner AB, Fitzpatrick TB. Inhibition of melanin
formation by chemical agents. J Invest Dermatol 1952;18:119-135.
10) Iijima S, Watanabe K. Studies on DOPA reaction. II. Effect
of chemicals on the reaction. J Invest Dermatol 1957;28:1-4.
11) Arnold J, Anthonioz P, Marchandd JP. Depigmenting action
of corticosteroids. Experimental study on guinea pigs. Dermatologica
1975;151:274-280.
12) Ortonne JP. Retinoic acid and pigment cells: a review
of in-vitro and in-vivo studies. Brit J Dermatol 1992;127(Suppl
41):43-7.
13) Garbe C, Karsagakis K, Kruger S, Orfanos CE. Potential
of different retinoids to induce cellular differentiation
of melanoma cells cultured in vitro. J Invest Dermatol 1991;96;1000.
14) Edward M, Gold JA, MacKie RM. Different susceptibilities
of melanoma cells to retinoic acid-induced changes in melanotic
expression. Biochem Biophys Res Commun 1988;155:773-778.
15) Lotan R. Lotan D. Enhancement of melanotic expression
in cultured mouse melanoma cells by retinoids. J Cell Physiol
1980;106:179-189.
16) Nakajima M, Shinoda I, Mikogami T, Iwamoto H, Hashimoto
S, Miyauchi H, Fukuwatari Y, Hayasawa H. À-Lactoglobulin
suppresses melanogenesis in cultured human melanocytes. Pigment
Cell Res 1997;10:410-413.
17) Clifford JL, Petkovich M, Chambon P, Lotan R. Moduation
by retinoids of mRNA levels for nuclear receptors in murine
melanoma cells. Mol Endocrinol 1990;4:1546-55.
18) Fligiel SE, Inman DR, Talwar HS, Fisher GJ, Voorhees
JJ, Varani J Modulation of growth in normal and malignant
melanocytic cells by all-trans retinoic acid. J Cutan Pathol
1992;19:27-33
19) Orlow SJ, Chakraborty AK, Pawelek JM. Retinoic acid is
a potent inhibitor of inducible pigmentation in murine and
hamster melanoma cell lines. J Invest Dermatol 1990;95:462-4.
20) Chakraborty AK, Orlow SJ, Pawelek JM. Stimulation of
the receptor for melanocyte-stimulating hormone by retinoic
acid. FEBS 1990;276:205-208.
21) Romero C, Aberdam E, Larnier C, Orthonne JP. Retinoic
acid as modulator of UVB-induced melanocyte differentiation.
Involvement of the melanogenic enzymes expression. J Cell
Sci 1994;107:1095-103.
22) Grabbe J, Welker P, Dippel E, Czarnetzki BM. Stem cell
factor, a novel cutaneous growth factor for mast cells and
melanocytes. Arch Dermatol Res 1994;287: 78-84.
23) Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott
G, Moellmann G, McGuire J. Basic fibroblast growth factor
from human keretinocytes is a natural mitogen for melanocytes.
J Cell Biol 1988;107:1611-9.
24) Matsumoto K, Tajima H, Nakamura T. Hepatocyte growth
factor is a potent stimulator of human melanocyte DNA synthesis
and growth. Biochem Biophys Res Commun 1991;176:45-51
25) Yada, Y, Higuchi K, Imokawa G. Effects of endothelins
on signal transduction and proliferation in human melanocytes.
J Biol Chem 1991;266:18352-18357.
26) Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic
mouse melanocytes, syngeneic with the B16 melanoma and requiring
a tumour promotor for growth. Int J Cancer 1987;39:414-8.
27) Ikeda T, Sai M, Fujiwara K, Honjoh T, Hashizume S. Serum-free
medium for normal human melanocytes. Animal Cell Technology:
Basic & Applied Aspects, 1993;6:345-349. (Proceedings
of the sixth international meeting of the Japanese association
for Animal Cell Technology, Nagoya, Japan, November 9-12,
1993)
28) Tsunenaga M, Kohno Y, Horii I, Yasumoto S, Huh N, Tachikawa
T, Yoshiki S, Kuroki T. Growth and differentiation properties
of normal and transformed human keratinocytes in organotypic
culture. Jpn J Cancer Res 1994;85:238-44.
29) Oikawa A., Nakayasu M. Qunatitative measurement of melanin
as tyrosinase equivalents and as weight of purified melanin.
Yale J Biol Med 1973;46:500-507.
30) Virador VM, Kobayashi N, Matsunaga J, Hearing VJ. A standardized
protocol for assessing regulators of pigmentation. Anal Biochem
1999;270:207-19.
31) Kligman, AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin
for photoaged skin. J Am Acad Dermatol 1986;15:836-59.
32) Eichner R. pidermal effects of retinoids: in vitro studies.
J Am Acad Dermatol 1986;15:789-97.
33) Fisher GJ, Voorhees JJ. Molecular mechanism of retinoid
actions in skin. FASEB J 1996;10:1002-13.
34) Welsh BM, Mason RS, Halliday GM. Topical all-trans retinoic
acid augments ultraviolet radiation-induced increases in
activated melanocyte numbers in mice. J Invest Dermatol 1999;112:271-8.
35) Sanquer, S, Reenstra WR, Eller MS, Gilchrest BA. Keratinocytes
and dermal actors activate CRABP-I in melanocytes. Exp Dermatol
1998;7:369-79.
36) Schwartz, E, Cruickchank FA, Mezick JA, Kligman LH. Topical
all-trans retinoic acid stimulates collagen synthesis in
vivo. J Invest Dermatol 1991;96:975-8.
37) Kligman, LH, Duo CH, Kligman AM. Topical retinoic acid
enhances the repair of ultraviolet damaged dermal connective
tisuue. Connect Tissue Res 1984;12:139-150.
38) Mcmichael AJ, Griffiths CEM, Talwar HS, Finkel LJ, Rafal
ES, Hamilton TA, Voorhees JJ. Concurrent application of tretinoin
(retinoic acid) partially protects against corticosteroid-induced
epidermal atrophy. Br J Dermatol 1996;135:60-64.
39) Lesnik RH, Mezick JA, Capetola RJ, Kligman LH. Topical
all-trans retinoic acid prevents corticosteroid-induced skin
atrophy without abrogating the anti-inflammatory effect.
J Am Acad Dermatol 1989;21:186-90.
40) Leyden, JL, Thaw M, Kligman AM. Steroid rosacea. Arch
Dermatol 1974;110:619-622.
41) Ulland AE, Shearer JD, Coulter C, Caldwell MD.Altered
wound healing arginine metabolism by corticosterone and retinoic
acid. J Surg Res 1997;70:84-6.
Legends
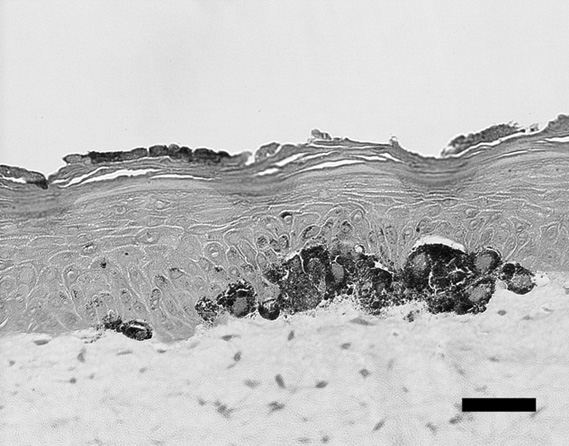
Fig. 1. Histological view of pigmented skin equivalents (one
of the control samples) at the site of intense pigmentation.
Bar = 50 Êm
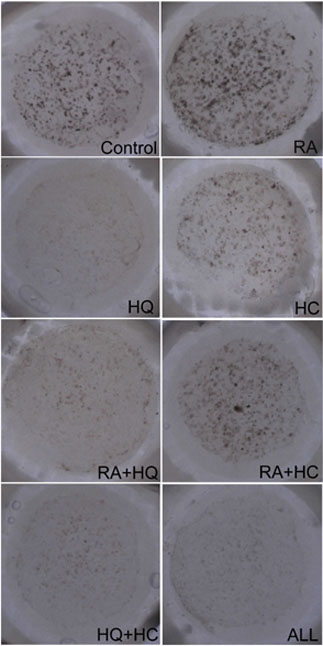
Fig. 2. Representative
samples of pigmented skin equivalents. Pigmented
spots were produced on the dermal equivalents inside
the glass rings (12 mm in diameter).
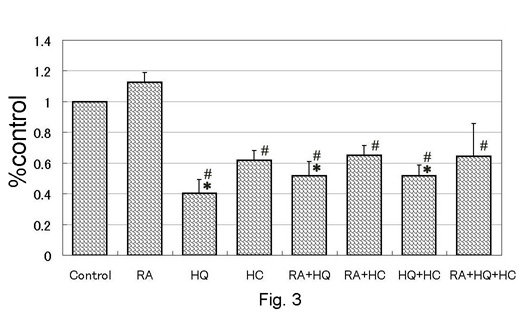
Fig. 3. Melanin production
on pigmented skin equivalents measured with a computed
image analyser. Values were shown as mean + standard
error. *: p<0.05 to control, #: p<0.05 to
RA.
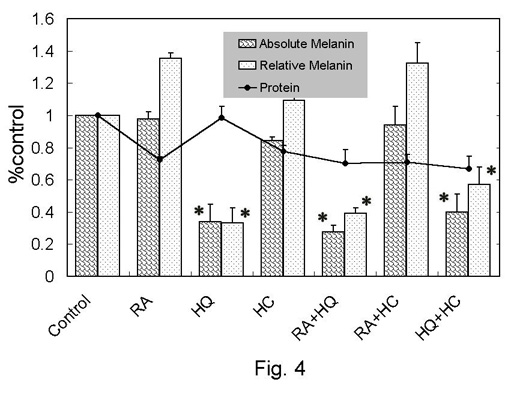
Fig. 4. Melanin and
protein assay in monolayer culture of murine melanocytes
(melan-a). The relative values to control were
shown as mean + standard error. Relative melanin
was calculated as (absolute melanin)/(protein).
*: p<0.05 to control.
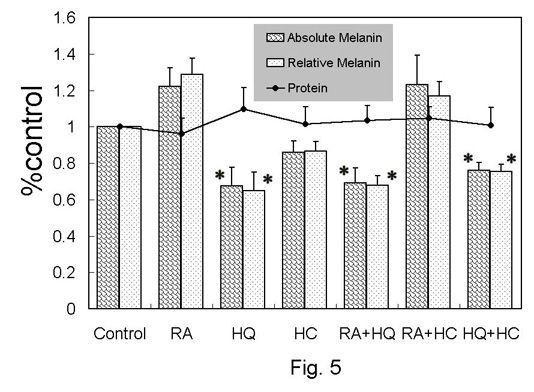
Fig. 5. Melanin and
protein assay in monolayer culture of human melanocytes.
The relative values to control were shown as mean
+ standard error. Relative melanin was calculated
as (absolute melanin)/(protein). *: p<0.05 to
control.
Fig. 6. Effects on tyrosinase
acitivity in monolayer culture of murine melanocytes
(melan-a).
Fig. 7. Effects on tyrosinase
acitivity in monolayer culture of human melanocytes.
@
|

