|
Introduction
The treatment of acne in post-adolescent females often
fails1,2, although there are a number of treatments
with a relative high degree of effectiveness. Some
patients suffer from severe and repetitive acne, and
others have widespread occurrences of acne such as
on the chest and back. Inflammation, scars, and post-inflammatory
hyperpigmentation impair their appearance. The treatment
of acne is based on two separate concepts: 1) anti-symptomatic
treatments such as the use of chemical peeling, lasers,
and antibiotics3 and 2) inhibitory treatments such
as the use of oral retinoids and anti-androgen therapies
(retinoids also have symptomatic effects). Systemic
treatments are especially needed for treating cases
with severe, repetitive, or the widespread type of
acne throughout the body.
Hormonal anti-androgenic treatments could be most
effective for post-adolescent females to inhibit sebum
production and acne formation, though they can induce
side effects such as menstrual irregularity. Cyproterone
acetate, a synthetic progestin, is the first steroidal
anti-androgen agent widely used in the treatment of
advanced prostatic carcinoma and severe acne. However,
it was reported that long-term use of cyproterone
acetate can induce severe hepatocellular dysfunction
and potential carcinogenesis4,5, and the sale of the
agent was discontinued in Japan in1999. It is still
widely used in single-use form or in combination with
ethinyl estradiol, a contraceptive (e.g. Diane-35),
for severe acne in many western countries. Flutamide
is a non-steroidal anti-androgenic agent, recognized
as a competitive agonist of the androgen receptor
and a beneficial compound for the treatment of prostate
cancer, benign prostatic hyperplasia, and hirsutism.
Despite its proven validity, hepatotoxicity due to
this agent has been reported and the use of this agent
is restricted6. In addition, flutamide inducing hepatotoxicity
is reported to be higher in Asians than in Caucasians7.
For this reason, in Japan, monthly blood tests for
checking liver function are required during the use
of flutamide. Spironolactone is well known as a diuretic
and has been used for over 20 years as a representative
antagonist of androgen in the treatment of acne and
hirsutism8,9. There have been several randomized studies
evaluating the efficacy of these agents in the treatment
of hirsutism10-13. Serious adverse drug-related experiences
and the discontinuation of therapy have been uncommon
in spironolactone therapy14, although gynecomastia15
and menstrual irregularities as side effects are frequently
seen accompanying high-dose use of spironolactone.
Other adverse side effects, such as lethargy, fatigue,
dizziness, or headache, were reported8,9,16,17.
As is well known, hormonal actions, serum hormone
levels, and side effects of hormonal agents frequently
differ among races or populations. As far as the Oriental
population is concerned, spironolactone appears to
be safest for treating acne among the three agents
discussed above with anti-androgenic action. The present
study was performed in order to assess the therapeutic
and side effects of oral spironolactone in Japanese
patients.
Patients and Methods
Spironolactone was administered orally to 139 Japanese
patients (ages 15 to 46 years, average 26.0 ± 6.8;
116 female and 23 male) suffering from acne at Ritz
Medical Clinic. Informed consent approved by IRB was
obtained from each patient or legal guardian. The
uses of other therapies for acne were not permitted
for the duration of the study. A 20-week course of
therapy was designed; the initial dose was 200 mg/day
for the first 8 weeks. After that, the doses were
progressively decreased by 50 mg every 4 weeks to
150 mg/day, 100 mg/day, and 50 mg/day (Fig.1).
The effectiveness of this treatment was evaluated
from the clinical results of 64 patients (all female)
who completed the 20-week treatment. A digital camera
was used to take clinical photographs before, during,
and after treatment. The therapeutic results were
graded according to the Consensus Conference on Acne
Classification18. Evaluations of improvement were
undertaken before and after treatment by two experienced
plastic surgeons who did not perform this treatment.
The investigators assessed each patient from his or
her photographs using a 3-point scale (2 = excellent
improvement, 1 = good improvement, and 0 = poor improvement).
In order to find related adverse events, we tested
the blood of the patients and collected before-and-after
data from 25 patients. Alanine transferase (ALT),
aspartate transferase (AST), blood urea nitrogen (BUN),
creatinine (Cr), Na+, K+, Cl-, luteinizing hormone
(LH), dehydroepiandrostenedione sulfate (DHEAS), sex
hormone binding globulin (SHBG), total testosterone
(T), and free T in serum were measured, although not
all of the data were measured from all patients due
to the shortage of blood collected from some patients.
Statistical Analysis
Data were reported as mean ± standard error. The nonparametric
Mann-Whitney U-test was used to compare clinical and
hormonal data from before and after treatment
Results
All 64 patients evaluated exhibited clinical improvement
(Table 1). Thirty-four patients (53.1%) showed fewer
inflammatory spots (grade 2; Figs. 2, 3 and 4). Thirty
patients (46.9%) showed a decrease in new acne papules
(grade 1).
On the subject of side effects and complications,
about 80% of 116 female patients suffered from menstrual
irregularities. Five patients had no menstrual bleeding
during the first 3 months of treatment, and four had
severe menstrual irregularities. Both of these were
therefore given an intramuscular injection of estrogen
and progesterone to induce menstrual bleeding.
Among the 23 male patients, 3 (13%) showed gynecomastia
after 4 to 8 weeks of treatment (all 3 patients were
between 19 and 21 years old). Therefore, the administration
of spironolactone was discontinued to all male patients.
Other adverse side effects that had previously been
reported such as lethargy, fatigue, dizziness, or
headache were not observed in this trial. Changes
in urinary frequency were occasionally observed, however
these did not seem to reduce the patients’ quality
of life. Other side effects occurred in only a few
patients: drug eruptions were seen in three of 139
patients and edema in the lower extremities was also
seen in three.
We analyzed the changes in serum hormones, laboratory
data, and blood levels of electrolytes resulting from
oral spironolactone treatment (Fig. 5). Regarding
testosterone (T) values, only a small number of patients
showed a high total T or free T level before treatment,
and a number of patients showed a low serum level
of total T or free T. There were no particular trends
in total T or free T before and after treatment, but
some patients with high total T or free T before treatment
tended to normalize their value after the treatment.
Similarly, patients with low total T or free T showed
a tendency to elevate their hormonal levels. The values
of LH, DHEAS, and SHBG of almost all patients were
within normal limits.
Indices of liver functions (ALT and AST) and those
of kidney functions (BUN and Cre) were not affected
by oral spironolactone. Unexpectedly, Na+ and K+ were
also not significantly affected by this urinary agent.
Only Cl- showed a significant decrease (p<0.05)
compared with the pretreatment value, however almost
all values were within normal limits.
Discussion
A key in treating acne, which almost always recurs,
is to perform both a preventive treatment as well
as a curative one. The results showed that oral spironolactone
is very effective for treating acne in Japanese females
without any serious side effects other than menstrual
irregularities.
There were no significant abnormalities in the pretreatment
data of serum TST and free TST in Japanese patients
suffering from severe acne. However, in this trial,
all female patients exhibited clinical improvement
in their acne after at least 4 months (usually 2 months
was sufficient) administration of oral spironolactone
without any other combined treatments. Thus, the results
strongly suggest that androgen signals affect acne
in almost all patients and factors other than serum
hormonal levels such as the expression level of androgen
receptors in the sebaceous glands have a critical
influence on sebum production and subsequent new acne
formation. The hypothesis may explain why some acne
patients do not respond to oral contraceptive treatments.
Oral contraceptives can reduce gonadotropins such
as LH and thereafter reduce T production and serum
level of T, but cannot block androgen signals at the
level of the androgen receptors. Thus, it is suggested
that oral spironolactone is advantageous for treating
acne compared with oral contraceptives. Gradually
decreasing spironolactone dosage can contribute to
preventing a recurrence of acne formation after the
treatment. Recurrences of acne occasionally occurred
several months after cessation of the administration
of oral spironolactone.
Some patients who also suffered from seborrheic dermatitis
on their faces showed improvement after the administration
of oral spironolactone. This probably results from
the inhibitory action of spironolactone for sebum
production by blocking androgen signals at the receptor
level. Indeed, almost all patients reported reduced
sebum discharge as soon as 2 weeks after starting
the oral spironolactone.
The mechanism of menstrual irregularities caused by
oral spironolactone is not clearly understood. After
the start of oral spironolactone administration, the
serum levels of T or dehydrotestosterone (DHT) could
be acutely elevated because androgen receptors are
competitively blocked by spironolactone. The excessive
T could then be transformed into estradiol, which
can induce endometrial hyperplasia and menstrual irregularities.
Gynecomastia can be induced in men for the same reason15.
As the 3 male patients with gynecomastia in this study
were between 19 and 22 years old, it is suggested
that this age range is most susceptible to suffer
from conversion from T to estradiol because of the
high serum level of T during this period. Oral treatment
with isotretinoin can be one important alternative
for male patients with severe acne19,20.
Most patients in our trial were satisfied with the
clinical results in spite of the menstrual irregularities
because they had been suffering from repetitive acne
for a long time and had lived a stressful life caused
by disfigurement. For 9 of 64 (14%) patients, a combined
injection of estrogen and progesterone was performed
for an effective induction of normal menstruation,
which was usually observed within 10 to 14 days after
the injection. Combined administration of contraceptives
can be useful for preventing possible pregnancy during
the treatment.
Other side effects such as itching and allergic eruptions
occurred in only a few patients. There were no abnormal
blood levels of electrolytes and laboratory data such
as ALT, AST, BUN, Cr, Na+, and K+ after administration
of the series of oral spironolactone. Only Cl- showed
a significant but minor decrease.
In summary, this study demonstrated that oral spironolactone
is quite effective for any type of acne in Asian females
and that this therapy is a good option, especially
for repetitive, severe, resistant, or widespread types
of acne in spite of its side effects such as menstrual
irregularities. Other additional therapies might be
helpful but not necessary for inhibiting new outbreaks
of acne. Male patients require an alternative treatment
such as oral isotretinoin. Oral spironolactone has
been used as a diuretic for a long time and its safety
has already been established for Asian people, although
two other hormonal agonists of acne therapy, cyproterone
acetate and flutamide, showed liver dysfunction in
Asians. Menstrual disturbance is common as an adverse
effect, but a compensatory hormonal injection is available
for inducing menstrual regularity.
References
1) Thiboutot D, Chen W. Update and future of hormonal
therapy in acne. Dermatology 2003;206,57-67.
2) Goulden V, Clark SM, Cunliffe WJ. Post-adolescent
acne: a review of clinical features. Br J Dermatol
1997;136: 66-70.
3) Zouboulis CC, Martin JP. Update and future systemic
acne treatment. Dermatology 2003;206:37-53.
4) Parys BT, Hamid S, Thomson RG. Severe hepatocellular
dysfunction following cyproterone acetate therapy.
Br J Urol 1991;67:312-3.
5) Kasper P. Cyproterone acetate: a genotoxic carcinogen?
Pharmacol Toxicol 2001;88:223-31.
6) Wysowski DK, Freiman JP, Tourtelot JB, Horton ML
3rd. Fatal and nonfatal hepatotoxicity associated
with flutamide. Ann Intern Med 1993;118:860-4.
7) Takashima E, Iguchi K, Usui S, Yamamoto H, Hirano
K. Metabolite profiles of flutamide-induced hepatic
dysfunction. Biol Pharm Bull 2003;26:1455-60.
8) Muhlemann MF, Carter GD, Cream JJ, Wise P. Oral
spironolactone: an effective treatment for acne vulgaris
in women. Br J Dermatol 1986;115:227-32.
9) Messina M, Manieri C, Musso MC, Pastorino R. Oral
and topical spironolactone therapies in skin androgenization.
Panminerva Med 1990;32:49-55.
10) Lucky AW, Mcguire J, Rosenfield RL, Lucky PA,
Rich BH. Plasma androgens in women with acne vulgaris.
J Invest Dermatol 1983;81:70-4.
11) Vexiau P, Husson C, Chivot M, Brerault JL, Fiet
J, Julien R, et al. Androgen excess in women with
acne alone compared with women with acne and/or hirsutism.
J Invest Dermatol 1990;94:279-83.
12) Carmina E, Stanczyk FZ, Matteri RK, Lobo RA. Serum
androsterone conjugates differentiate between acne
and hirsutism in hyperandrogenic women. Fertil Steril
1991;55:872-6.
13) Slayden SM, Moran C, Sams WM Jr, Boots LR, Azziz
R. Hyperandrogenemia in patients presenting with acne.
Fertil Steril 2001;75:889-92.
14) Shaw JC, White LE. Long-term safety of spironolactone
in acne: results of an 8-year followup study. J Cutan
Med Surg 2002;6:541-5.
15) Satoh T, Itoh S, Seki T, Itoh S, Nomura N, Yoshizawa
I. On the inhibitory action of 29 drugs having side
effect gynecomastia on estrogen production. J Steroid
Biochem Mol Biol 2002;82:209-16.
16) Masahashi T, Wu MC, Ohsawa M, Asai M, Ichikawa
Y, Hanai K, et al. Spironolactone therapy for hyperandrogenic
anovulatory women--clinical and endocrinological study.
Nippon Sanka Fujinka Gakkai Zasshi 1996;38:95-101.
17) Shaw JC. Low-dose adjunctive spironolactone in
the treatment of acne in women: a retrospective analysis
of 85 consecutively treated patients. J Am Acad Dermatol
2000;43:498-502.
18) Pochi PE, Shalita AR, Strauss JS, Webster SB,
Cunliffe WJ, Katz HI, et al. Report of the Consensus
Conference on Acne Classification. Washington, D.C.,
March 24 and 25, 1990.J Am Acad Dermatol 1991;24:495-500.
19) Ng PP, Goh CL. Treatment outcome of acne vulgaris
with oral isotretinoin in 89 patients. Int J Dermatol
1999;38:213-6.
20) Cooper AJ; Australian Roaccutane Advisory Board.
Treatment of acne with isotretinoin: recommendations
based on Australian experience. Australas J Dermatol
2003;44:97-105.
Legends
Figure 1. Protocol of oral spironolactone administration.
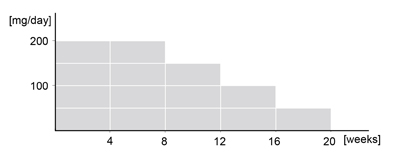
Figure 2. 27-year-old female with
acne vulgaris from the jawline to the neck who did
not improve by repeated AHA peeling and oral antibiotics.
(a) Before treatment. (b) After 20 weeks of oral spironolactone
(grade2, fewer inflammatory spots).
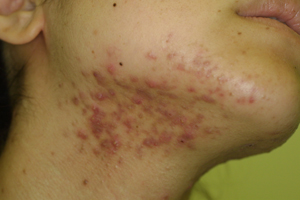
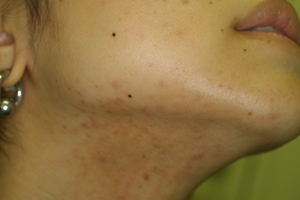
Figure 3. 31-year-old female with
acne vulgaris and seborrheic dermatitis on the whole
face. (a) Before treatment. (b) After 4 months of
oral spironolactone (grade 2). Sebum discharge was
markedly reduced and seborrheic dermatitis and acne
were both improved.
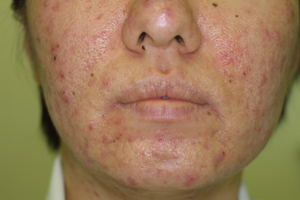
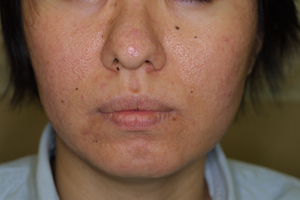
Figure 4. 28-year-old female with
acne vulgaris and severe sebum discharge. (a) Before
treatment. (b) After 4 months of oral spironolactone
(grade 2).
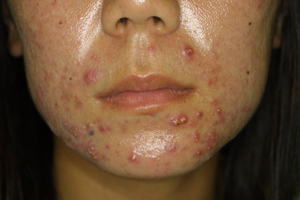
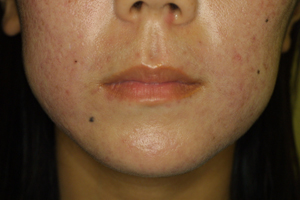
Figure 5. Data of blood tests before
and after administration of oral spironolactone: (a)
Total T, (b) Free T, (c) LH, (d) DHEAS, (e) SHBG,
(f) ALT, (g) AST, (h) BUN), (i) Cre, (j) Na+, (k)
K+, and (l) Cl-. Increased or decreased data by more
than 10 % of initial values were shown as blue or
red lines, respectively. Free testosterone showed
significant increases (p<0.05) and Cl- showed significant
decreases (p<0.05) compared with pretreatment values,
however almost all values were within normal limits.
LH, DHEAS, SHBG, ALT, AST, BUN, Cre, Na+, and K+ did
not show any significant differences.
|

