|
Human adherent stromal cells isolated from adipose
tissue have been shown to have multipotency [1, 2],
able to differentiate not only into mesenchymal lineages
including endothelial cells [3-6] and cardiomyocytes
[7-9] but also into neural cells [2] and hepatocytes
[10]. These cells have been referred to by various
names, including preadipocytes, vascular stromal cells,
adipose-derived mesenchymal progenitor cells, and
adipose stromal cells. In this study, we refer to
the cells as adipose-derived stem cells (ASCs). The
characteristics of ASCs have been extensively studied
[11-15], as well as the potential clinical applications
of ASCs [3-7, 16]. In addition, clinical trials have
already begun involving enhancement of bone and adipose
regeneration and angiogenesis [17-20].
Adipose tissue is thought to be a promising source
of stem cells because it can be harvested in relatively
large quantities (100 mL to > 1 L) using liposuction
with minimal morbidity. Although ASCs may be clinically
used without cell expansion because of their large
quantities, it is of great value to culture and expand
ASCs safely and effectively without losing their multipotency
for manipulation and further development of cell-based
therapies. There have been some reports indicating
enhanced proliferation of human ASCs using specific
culture media with supplements. It was shown that
fibroblast growth factor (FGF)-2 was released by ASCs,
enhanced proliferation [21-24], and maintained the
adipogenic potential of ASCs [21]. FGF-1 and epidermal
growth factor (EGF) were suggested to act as stimulators
of both ASC proliferation and differentiation [25-27].
Platelet -derived growth factor (PDGF)-BB [25, 28],
tumor necrosis factor (TNF)-α [29], and insulin-like
growth factor (IGF)-1 [29] were also shown to promote
ASC proliferation, and the former two factors were
suggested to have inhibiting effects on ASC differentiation
[25, 29]. However, it has not been shown whether ASCs
expanded by these methods preserve their multipotency
or not.
In this study, we investigated the effects of an endothelial
growth medium (EGM-2R, Cambrex, Walkersville, MD)
on culturing human ASCs, focusing on proliferation
and differentiation potentials. EGM-2 is usually used
to support the growth of endothelial cells. In recent
studies, EGM-2 has been used for culture of non-endothelial
cells [16, 30, 31]. However, ASCs have usually been
cultured in Dulbecco’s modified Eagle’s medium (DMEM)
or DMEM/F12 medium, and the effects of EGM-2 on ASCs
have not been reported.
Materials and methods
Cell isolation and culture
We obtained liposuction aspirates from 12 healthy
female donors undergoing liposuction of the abdomen
or thighs after informed consent using an IRB-approved
protocol. The stromal vascular fraction containing
ASCs was isolated from the fatty portion of liposuction
aspirates, as previously described [15]. Briefly,
the aspirated fat was washed with phosphate buffered
saline (PBS) and digested on a shaker at 37oC in PBS
containing 0.075% collagenase for 30 min. Mature adipocytes
and connective tissues were separated from pellets
by centrifugation (800×g, 10 min). The cell pellets
were resuspended, filtered with a 100-μm mesh (Millipore,
MA, USA), plated at a density of 5×105 nucleated cells/100-mm
dish, and cultured at 37°C in an atmosphere of 5%
CO2 in humid air. The culture medium was: (1) DMEM
(Nissui Pharmaceutical, Tokyo, Japan) containing 10%
fetal bovine serum (FBS), or (2) EGM-2 containing
2% FBS. Endothelial basal medium (EBM, Cambrex) is
a basal medium for EGM-2. EGM-2 does not contain any
animal-derived factors but does contain FGF-2, vascular
endothelial growth factor (VEGF), IGF-1, EGF, ascorbic
acid, hydrocortisone, GA-1000 (gentamicin and amphotericin-B),
and heparin, although the concentration of each agent
is not disclosed. Primary cells were cultured for
7 days and were defined as “Passage 0.” The medium
was replaced every 3 days. Cells were passaged every
week by trypsinization.
<Measurement of doubling time
and total cell number>
During cell culture in each medium, doubling time
was measured at passages 0, 1, 2, and 3 by seeding
ASCs (Passage 0) at a density of 1×105 cells per 10-cm
dish. After cells reached the logarithmic growth phase,
they were sequentially trypsinized every 48 h and
counted with a cell counter (NucleoCounter, Chemometec,
Allerod, Denmark). Doubling time was calculated according
to the following formula: doubling time = 48 h/log2(N2/N1),
where N1 is the first cell count and N2 is the cell
count 48 h later. Total cell number after the initiation
of culture in each medium was also measured by seeding
ASCs (Passage 0) at a density of 1×104 cells per 3.5-cm
dish and culturing the cells until they reached the
stationary phase.
Measurement of the proliferative
effect of supplemented growth factors
To examine the proliferative effect of each growth
factor supplemented in EGM-2, ASCs were cultured in
medium supplemented with a single growth factor (VEGF,
EGF, IGF-1, or FGF-2). EBM containing 2% FBS was used
as the control medium. ASCs cultured in the control
medium (Passage 0) were seeded at a density of 1×104
cells per well in a 6-well plate. Cells were cultured
in the control medium (2% FBS), supplemented medium
(0.1, 1, or 10 ng/ml of each growth factor) (2% FBS),
DMEM (10% FBS), or EGM-2 (2% FBS). The number of cells
after 7 days of culture was counted using a cell counter.
Flow cytometry of cultured cells
Cultured cells in each medium were examined for surface
marker expression using flow cytometry. The following
monoclonal antibodies (MAbs) conjugated to fluorochromes
were used: anti-CD29-PE, CD31-PE, CD34-PE, CD45-PE,
CD90-PE, CD146-PE (BD Biosciences, San Diego, CA),
CD105-PE (Serotec, Oxford, UK), and Flk-1-PE (Techne,
Minneapolis, MN). Control MAbs were included for all
fluorochromes. Cells were incubated with directly
conjugated MAbs for 30 minutes, then washed and fixed
in 1% paraformaldehyde. Cells were analyzed using
an LSR II (Becton Dickinson, San Jose, CA) flow cytometry
system. Data acquisition and analysis were then performed
(Cell Quest software, Becton Dickinson). Gates were
set based on staining with combinations of relevant
and irrelevant MAbs so that no more than 0.1% of cells
were positive using irrelevant antibodies.
Induced differentiation of cultured
cells
After culture in each medium for 2 weeks, differentiation
into the adipogenic, chondrogenic, and osteogenic
lineages was examined.
For adipogenic differentiation, cells were incubated
for 4 weeks in DMEM containing 10% FBS supplemented
with 0.5 mM isobutyl-methylxanthine (Sigma, St. Louis,
MO), 1 ?M dexamethasone, 10 ?M insulin (Sigma), and
200 ?M indomethacin. Adipogenic differentiation was
visualized with oil red O staining. For quantitative
analysis of lipid droplets, we measured Nile Red fluorescence,
using AdipoRedTM (Cambrex), with excitation at 485
nm and emission at 535 nm.
For chondrogenic differentiation, cells were incubated
for 4 weeks in DMEM containing 1% FBS supplemented
with 6.25 ?g/ml insulin, 10 ng/ml TGF-β1, and 50 nM
ascorbate-2-phosphate. Chondrogenic differentiation
was visualized with Alcian Blue staining. For quantitative
analysis, a micromass culture system was used as previously
reported [32]. Cells were plated in a 15-ml tube and
cultured in the chondrogenic medium for 3 weeks. Then,
the diameter of a micromass was measured.
For osteogenic differentiation, cells were incubated
for 4 weeks in DMEM containing 10% FBS supplemented
with 0.1 μM dexamethasone, 50 μM ascorbate-2-phosphate,
and 10 mM β-glycerophosphate (Nacalai Tesque, Kyoto,
Japan). Osteogenic differentiation was visualized
with von Kossa staining. For quantitative analysis
of total calcium, calcium deposition was evaluated
based on the ortho-cresolphthalein complexone (OCPC)
method with the Calcium C-Test Wako Kit (Wako Chemicals)
according to the manufacturer’s instructions.
Statistical analyses
Results were expressed as mean ± SEM. Welch’s t-test
was used to compare each parameter. A value of p <
0.05 was considered significant.
Results
Doubling time and total cell number
Doubling time of ASCs cultured with EGM-2 was significantly
shorter than that of cells cultured with DMEM at each
passage (19.3 ± 2.1 h vs. 39.8 ± 6.8 h at Passage
0; 15.6 ± 1.1 h vs. 55.1 ± 3.5 h at Passage 1; 20.3
± 0.7 h vs. 52.0 ± 2.4 h at Passage 2; and 26.5 ±
1.1 h vs. 54.3 ± 6.4 h at Passage 3) (Fig. 1A). Total
cell number showed that ASCs cultured with EGM-2 proliferated
more rapidly and reached the stationary phase earlier
than those cultured with DMEM (40 days vs. 200 days),
though the maximum population doubling level of ASCs
was similar either when cultured with EGM-2 or DMEM
(35?40 with EGM-2 vs. 40?45 with DMEM) (Fig. 1B).
Differentiation assays were performed using ASCs cultured
with each medium for 2 weeks, and at that stage, ASCs
cultured with EGM-2 were supposed to be expanded 105
times (1010 vs. 105) compared to those cultured with
DMEM (Fig. 1B).
Proliferative effect of each growth
factor
VEGF, EGF, and IGF-1 showed no significant proliferative
effect on ASCs cultured in EBM containing 2% FBS.
FGF-2 at a density of 0.1, 1, or 10 ng/ml significantly
promoted proliferation of ASCs compared to control.
However, the proliferative effect of FGF-2 was much
less than that of EGM-2 containing all of the growth
factors, indicating a synergistic effect of supplemented
growth factors (Fig. 2).
Flow cytometry
Flow cytometry of ASCs cultured in DMEM and EGM-2
showed no significant differences except CD 105 at
passages 1, 2, and 3 (Table 1). Both cell populations
uniformly expressed mesenchymal markers (CD29 and
CD90) and were devoid of a hematopoietic cell marker
CD45. Expressions of CD34 (stem cell marker), CD31
(endothelial cell marker), CD146 (endothelial and
mural cells marker), and Flk-1 (VEGFR-2) were similar
in both cell populations, and CD34 expression of ASCs
markedly decreased at passage 1 in both media (Fig.
3).
Differentiation capacity
Both cell populations cultured in DMEM and EGM-2 for
2 weeks had similar capacities to differentiate into
adipogenic, chondrogenic, and osteogenic lineages.
No morphological differences between the two cell
populations were observed during and after differentiation
(Fig. 4A). Quantitative analyses (lipid droplets in
adipogenic differentiation, micromass diameter in
chondrogenic differentiation, and total calcium content
in osteogenic differentiation) also showed no significant
differences between the two cell populations (Fig.
4B).
Discussion
In this study, EGM-2 expanded ASCs very rapidly while
preserving their multipotency for at least 2 weeks;
the proliferative efficiency of EGM-2 was 105 times
of that of DMEM in the first 2 weeks. A doubling time
of ASCs shorter than that shown in this study (15?20
hours) has not been reported previously in the literature.
EGM-2 contains 2% FBS and various growth factors including
FGF-2, VEGF, IGF-1, and EGF. The highly boosting effects
of EGM-2 on ASC proliferation are suggested to result
from supplemented growth factors and other unknown
synergistic effects, as discussed below. ASCs cultured
with EGM-2 proliferated much more rapidly and reached
the stationary phase earlier than those cultured with
DMEM (40 days vs. 200 days), although the maximum
population doubling levels were similar between the
two culture media (Fig. 1B). The results suggest that
a majority of ASCs may have a limited capacity of
self renewal.
Serum concentrations can affect proliferation activity
of ASCs. We previously reported that the doubling
time of ASCs cultured with 15% FBS was significantly
shorter than that of cells cultured with 10% FBS,
although the culture media was M199 supplemented with
FGF-1 in that study [15]. FBS is made through coagulations
of fetal bovine whole blood; thus, it is supposed
to contain not only IGF-1, which is regularly present
in serum including platelet-poor plasma-derived serum,
but also platelet-derived cytokines such as PDGF and
EGF [our unpublished data, in submission]. In the
present study, however, the doubling time of ASCs
cultured in DMEM was significantly longer than that
for ASCs cultured in EGM-2, in spite of the higher
FBS concentration (10%) in DMEM compared to EGM-2
(2%). This result suggests that the growth factors
added to EGM-2 have greater effects on the proliferation
activity of ASCs than do serum concentrations. In
fact, a recent report suggested that platelet-derived
growth factors (not designated but assumed to be PDGF
and EGF) may reduce proliferation activity and adipogenic
differentiation capacity of ASCs [23].
Among growth factors contained in EGM-2, supplementation
with IGF-1, EGF, or VEGF did not significantly promote
proliferation activity of ASCs in EBM culture containing
2% FBS. The growth factors have some proliferative
effects on ASCs when added to serum-free media or
SPPP, as reported previously [5, 23, 29]; however,
any effects in this study using a low concentration
of FBS with IGF-1 and EGF were subtle or masked. In
this study, only FGF-2 showed a statistically significant
promoting effect on ASC proliferation, which has been
suggested in previous studies [21-24]. The results
strongly suggest that FGF-2 is a critical growth factor
for supplementation of serum-containing culture media.
A previous study suggested that FGF-2 plays a critical
role in self renewal of ASCs [21]. It was also shown
that FGF-2 added to SPPP increased proliferation activity
and adipogenic differentiation capacity [23]. Another
study reported efficient proliferation of ASCs transfected
with the FGF-2 gene [33]. However, in 7 days, EGM-2
expanded ASCs significantly and several-fold more
compared to FGF-2-supplemented EBM (Fig. 2), and thus
the effect of EGM-2 on ASC proliferation cannot be
explained solely based on the influence of FGF-2 alone.
It is likely that synergistic effects of various growth
factors and other factors contributed to the exceptional
efficiency.
In our study, the character of ASCs expanded with
EGM-2 did not appear to change significantly. ASCs
cultured with EGM-2 preserved differentiation capacities
similar to those with DMEM at least into three mesenchymal
lineages: adipogenic, chondrogenic, and osteogenic.
In addition, flow cytometry of both populations showed
no significant differences except for CD105 and presented
no increase of differentiation markers such as CD31,
suggesting that ASCs remain in an undifferentiated
and proliferating state. These results suggest that
EGM-2 accelerates expansion of ASCs mainly by facilitating
proliferation of undifferentiated cells.
Recent reports have shown that ASCs can differentiate
into endothelial cells in vitro under certain culture
conditions using endothelial growth media and also
in vivo [3-6]. ASCs may be essentially common progenitors
of adipocytes and vascular cells [5]. In most of the
in vitro studies, a semisolid medium like methylcellulose
or Matrigel was used, which may be key to endothelial
differentiation of ASCs [34]. Although EGM-2 containing
VEGF was originally a medium for expanding endothelial
cells and ASCs express Flk-1, a VEGF receptor, endothelial
cell marker expression of ASCs was not enhanced by
EGM-2 in our study using cell culture on a plastic
dish. In addition, hypoxic conditions have various
influences on ASCs [35-38], one of which is enhancing
ASC secretion of angiogenic factors such as VEGF and
HGF [35]. In the studies showing endothelial differentiation
of ASCs in vivo, ASCs were transplanted to the ischemic
hindlimb or under other ischemic conditions [4-6],
so that a hypoxic condition may be an important factor
in endothelial differentiation in vivo.
A number of preclinical studies with human ASCs have
been reported; in most, undifferentiated ASCs were
used, rather than ASCs differentiated into a specific
lineage, although the functional mechanism of transplanted
ASCs varied among studies. Transplanted ASCs survive
as undifferentiated cells and act as tissue-specific
progenitors or provider cells of soluble factors in
some studies [3, 7, 16]. In others, transplanted ASCs
differentiated into a specific lineage such as bone
and vessels according to the circumstances of recipient
sites [3, 7, 17]. In the therapeutic use of ASCs,
expansion of undifferentiated cells, rather than their
differentiation into a specific lineage, is likely
of great importance in the processing of the cells
before transplantation. In clinical practice, a safer
and more rapid expansion method is required in view
of time and cost requirements. EGM-2 does not contain
animal-derived factors, and the FBS used in this study
can be easily replaced with autologous serum or human
allogenic serum. The present expansion method with
EGM-2 has an exceptional efficiency and lays the groundwork
for establishing a practical route to mega-expansion
of ASCs for clinical applications.
Acknowledgments
We are very grateful to Ayako Kurata, Akiko Matsuura,
and Satomi Kawarasaki for their technical assistance.
References
[1] P.A. Zuk, M. Zhu, H. Mizuno, J. Huang, J.W. Futrell,
A.J. Katz, P. Benhaim, H.P. Lorenz, M.H. Hedrick,
Multilineage cells from human adipose tissue: implications
for cell-based therapies, Tissue Eng. 7 (2001) 211-228.
[2] P.A. Zuk, M. Zhu, P. Ashjian, D.A. De Ugarte,
J.I. Huang, H. Mizuno, Z.C. Alfonso, J.K. Fraser,
P. Benhaim, M.H. Hedrick, Human adipose tissue is
a source of multipotent stem cells, Mol. Biol. Cell
13 (2002) 4279-4295.
[3] D. Matsumoto, K. Sato, K. Gonda, Y. Takaki, T.
Shigeura, T. Sato, E. Aiba-Kojima, F. Iizuka, K. Inoue,
H. Suga, K. Yoshimura, Cell-assisted lipotransfer:
supportive use of human adipose-derived cells for
soft tissue augmentation with lipoinjection, Tissue
Eng. 12 (2006) 3375-3382.
[4] V. Planat-Benard, J.S. Silvestre, B. Cousin, M.
Andre, M. Nibbelink, R. Tamarat, M. Clergue, C. Manneville,
C. Saillan-Barreau, M. Duriez, A. Tedgui, B. Levy,
L. Penicaud, L. Casteilla, Plasticity of human adipose
lineage cells toward endothelial cells: physiological
and therapeutic perspectives, Circulation 109 (2004)
656-663.
[5] A. Miranville, C. Heeschen, C. Sengenes, C.A.
Curat, R. Busse, A. Bouloumie, Improvement of postnatal
neovascularization by human adipose tissue-derived
stem cells, Circulation 110 (2004) 349-355.
[6] Y. Cao, Z. Sun, L. Liao, Y. Meng, Q. Han, R.C.
Zhao, Human adipose tissue-derived stem cells differentiate
into endothelial cells in vitro and improve postnatal
neovascularization in vivo, Biochem. Biophys. Res.
Commun. 332 (2005) 370-379.
[7] Y. Miyahara, N. Nagaya, M. Kataoka, B. Yanagawa,
K. Tanaka, H. Hao, K. Ishino, H. Ishida, T. Shimizu,
K. Kangawa, S. Sano, T. Okano, S. Kitamura, H. Mori,
Monolayered mesenchymal stem cells repair scarred
myocardium after myocardial infarction, Nat. Med.
12 (2006) 459-465.
[8] J.K. Fraser, R. Schreiber, B. Strem, M. Zhu, Z.
Alfonso, I. Wulur, M.H. Hedrick, Plasticity of human
adipose stem cells toward endothelial cells and cardiomyocytes,
Nat. Clin. Pract. Cardiovasc. Med. Suppl 1 (2006)
33-37.
[9] V. Planat-Benard, C. Menard, M. Andre, M. Puceat,
A. Perez, J.M. Garcia-Verdugo, L. Penicaud, L. Casteilla,
Spontaneous cardiomyocyte differentiation from adipose
tissue stroma cells. Circ. Res. 94 (2004) 223-229.
[10] M.J. Seo, S.Y. Suh, Y.C. Bae, J.S. Jung, Differentiation
of human adipose stromal cells into hepatic lineage
in vitro and in vivo. Biochem. Biophys. Res. Commun.
328 (2005) 258-264.
[11] S. Gronthos, D.M. Franklin, H.A. Leddy, P.G.
Robey, R.W. Storms, J.M. Gimble, Surface protein characterization
of human adipose tissue-derived stromal cells, J.
Cell. Physiol. 189 (2001) 54-63.
[12] A.J. Katz, A. Tholpady, S.S. Tholpady, H. Shang,
R.C. Ogle, Cell surface and transcriptional characterization
of human adipose-derived adherent stromal (hADAS)
cells, Stem Cells 23 (2005) 412-423.
[13] S.S. Tholpady, R. Llull, R.C. Ogle, J.P. Rubin,
J.W. Futrell, A.J. Katz, Adipose tissue: stem cells
and beyond, Clin. Plast. Surg. 33 (2006) 55-62.
[14] C. Sengenes, K. Lolmede, A. Zakaroff-Girard,
R. Busse, A. Bouloumie, Preadipocytes in the human
subcutaneous adipose tissue display distinct features
from the adult mesenchymal and hematopoietic stem
cells, J. Cell. Physiol. 205 (2005) 114-122.
[15] K. Yoshimura, T. Shigeura, D. Matsumoto, T. Sato,
Y. Takaki, E. Aiba-Kojima, K. Sato, K. Inoue, T. Nagase,
I. Koshima, K. Gonda, Characterization of freshly
isolated and cultured cells derived from the fatty
and fluid portions of liposuction aspirates, J. Cell.
Physiol. 208 (2006) 64-76.
[16] H. Nakagami, K. Maeda, R. Morishita, S. Iguchi,
T. Nishikawa, Y. Takami, Y. Kikuchi, Y. Saito, K.
Tamai, T. Ogihara, Y. Kaneda, Novel autologous cell
therapy in ischemic limb disease through growth factor
secretion by cultured adipose tissue-derived stromal
cells, Arterioscler. Thromb. Vasc. Biol. 25 (2005)
2542-2547.
[17] S. Lendeckel, A. Jodicke, P. Christophis, K.
Heidinger, J. Wolff, J.K. Fraser, M.H. Hedrick, L.
Berthold, H.P. Howaldt, Autologous stem cells (adipose)
and fibrin glue used to treat widespread traumatic
calvarial defects: case report, J. Craniomaxillofac.
Surg. 32 (2004) 370-373.
[18] D. Garcia-Olmo, M. Garcia-Arranz, D. Herreros,
I. Pascual, C. Peiro, J.A. Rodriguez-Montes, A phase
I clinical trial of the treatment of Crohn’s fistula
by adipose mesenchymal stem cell transplantation,
Dis. Colon. Rectum 48 (2005) 1416-1423.
[19] K. Yoshimura, D. Matsumoto, K. Gonda, A clinical
trial of soft tissue augmentation by lipoinjection
with adipose-derived stromal cells (ASCs). Proceedings
of the 8th annual meeting of Tissue Engineering Society
International (TESI), pp206-207, Shanhai, China, 2005.
[20] T.A. Moseley, M. Zhu, M.H. Hedrick, Adipose-derived
stem and progenitor cells as fillers in plastic and
reconstructive surgery. Plast. Reconstr. Surg. 118
(2006) 121S-128S.
[21] L. Zaragosi, G. Ailhaud, C. Dani, Autocrine fibroblast
growth factor 2 signaling is critical for self-renewal
of human multipotent adipose-derived stem cells, Stem
Cells 24 (2006) 2412-2419.
[22] N. Quarto, M.T. Longaker, FGF-2 inhibits osteogenesis
in mouse adipose tissue-derived stromal cells and
sustains their proliferative and osteogenic potential
state, Tissue Eng. 12 (2006) 1-14.
[23] E. Koellensperger, D.V. Heimburg, M. Markowicz,
N. Pallua, Human serum from platelet-poor plasma for
the culture of primary human preadipocytes, Stem Cells
24 (2006) 1218-1225.
[24] M. Chiou, Y. Xu, M.T. Longaker, Mitogenic and
chondrogenic effects of fibroblast growth factor-2
in adipose-derived mesenchymal cells, Biochem. Biophys.
Res. Commun. 343 (2006) 644-652.
[25] H. Hauner, K. Rohrig, T. Petruschke, Effects
of epidermal growth factor (EGF), platelet-derived
growth factor (PDGF) and fibroblast growth factor
(FGF) on human adipocyte development and function,
Eur. J. Clin. Invest. 25 (1995) 90-96.
[26] L. Hutley, W. Shurety, F. Newell, R. McGeary,
N. Pelton, J. Grant, A. Herington, D. Cameron, J.
Whitehead, J. Prins, Fibroblast growth factor 1: a
key regulator of human adipogenesis. Diabetes 53 (2004)
3097-3106.
[27] G. Serrero, EGF inhibits the differentiation
of adipocyte precursors in primary cultures. Biochem.
Biophys. Res. Commun. 146 (1987) 194-202.
[28] Y.J. Kang, E.S. Jeon, H.Y. Song, J.S. Woo, J.S.
Jung, Y.K. Kim, J.H. Kim, Role of c-Jun N-terminal
kinase in the PDGF-induced proliferation and migration
of human adipose tissue-derived mesenchymal stem cells,
J. Cell. Biochem. 95 (2005) 1135-1145.
[29] K.M. Kras, D.B. Hausman, R.J. Martin, Tumor necrosis
factor-? stimulates cell proliferation in adipose
tissue-derived stromal-vascular cell culture: promotion
of adipose tissue expansion by paracrine growth factors,
Obes. Res. 8 (2000) 186-193.
[30] D. Simper, P.G. Stalboerger, C.J. Panetta, S.
Wang, N.M. Caplice, Smooth muscle progenitor cells
in human blood, Circulation 106 (2002) 1199-1204.
[31] R. Zhang, H. Yang, M. Li, Q. Yao, C. Chen, Acceleration
of endothelial-like cell differentiation from CD14+
monocytes in vitro, Exp. Hematol. 33 (2005) 1554-1563.
[32] B. Johnstome, T.M. Hering, A.I. Caplan, V.M.
Goldberg, J.U. Yoo, In vitro chondrohenesis of bone
marrow-derived mesenchymal progenitor cells, Exp.
Cell. Res. 238 (1998) 265-272.
[33] H. Yamashiro, T. Inamoto, M. Yagi, M. Ueno, H.
Kato, M. Takeuchi, S. Miyatake, Y. Tabata, Y. Yamaoka,
Efficient proliferation and adipose defferentiation
of human adipose tissue-derived vascular stromal cells
transfected with basic fibroblast growth factor gene,
Tissue Eng. 9 (2003) 881-892.
[34] U.M. Gehling, S. Ergun, U. Schumacher, C. Wagener,
K. Pantel. M. Otte, G. Schuch, P. Schafhausen, T.
Mende, N. Kilic, K. Kluge, B. Schafer, D.K. Hossfeld,
W. Fiedler, In vitro differentiation of endothelial
cells from AC133-positive progenitor cells, Blood
95 (2000) 3106-3112.
[35] J. Rehman, D. Traktuev, J. Li, S. Merfeld-Clauss,
C.J. Temm-Grove, J.E. Bonvenkerk, C.L. Pell, B.H.
Johnstone, R.V. Considine, K.L. March, Secretion of
angiogenic and antiapoptotic factors by human adipose
stromal cells, Circulation 109 (2004) 1292-1298.
[36] K.H. Kim, M.J. Song, J. Chung, H. Park, J.B.
Kim, Hypoxia inhibits adipocyte differentiation in
a HDAC-independent manner, Biochem. Biophys. Res.
Commun. 333 (2005) 1178-1184.
[37] D.W. Wang, B. Fermor, J.M. Gimble, H.A. Awad,
F. Guilak, Influence of oxygen on the proliferation
and metabolism of adipose derived adult stem cells,
J. Cell. Physiol. 204 (2005) 184-191.
[38] J.H. Lee, D.M. Kemp, Human adipose-derived stem
cells display myogenic potential and perturbed function
in hypoxic conditions, Biochem. Biophys. Res. Commun.
341 (2006) 882-888.
Figure legends
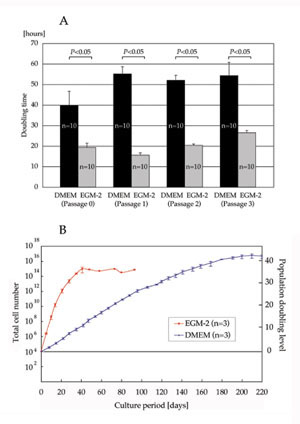
Fig. 1. (A) Doubling time at passages 0?3. Doubling
time of ASCs cultured with EGM-2 was significantly
shorter than for those cultured with DMEM at each
passage.
(B) Total cell number and population doubling level
after the initiation of culture with DMEM or EGM-2.
ASCs cultured in EGM-2 proliferated more rapidly and
reached the stationary phase earlier than those cultured
in DMEM.
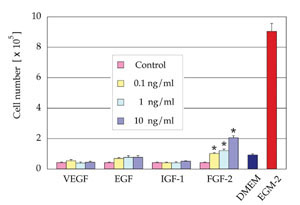
Fig. 2. Cell number after 7 days of culture in EBM
(2% FBS) supplemented with one of the following growth
factors, VEGF, EGF, IGF-1, or FGF-2 (n=3). FGF-2 at
a density of 0.1, 1, or 10 ng/ml significantly promoted
proliferation of ASCs compared to control medium.
The numbers of cells cultured in DMEM (10% FBS) or
EGM-2 (2% FBS) are also indicated. *: p < 0.05.
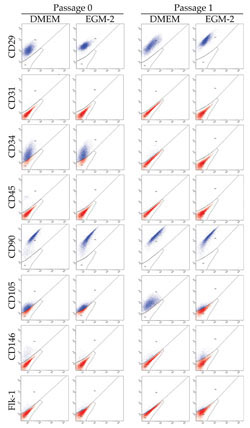
Fig. 3. Representative results of flow cytometry at
Passage 0 and Passage 1. No significant differences
were observed between the two cell populations cultured
in DMEM and EGM-2 except for CD 105 at Passage 1.
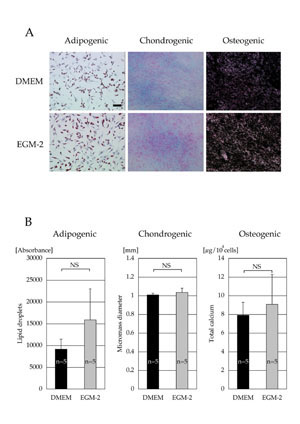
Fig. 4. (A) Microscopic results of cell differentiation.
Both cell populations cultured in DMEM and EGM-2 for
2 weeks had similar capacities to differentiate into
adipogenic, chondrogenic, and osteogenic lineages.
Adipogenic, chondrogenic, and osteogenic differentiations
were visualized with oil red O staining, Alcian Blue
staining, and von Kossa staining, respectively. Scale
bar = 100 μm.
(B) Quantitative analyses of cell differentiation.
Differentiation potentials were evaluated by lipid
droplet contents (adipogenic), micromass diameter
(chondrogenic), and total calcium contents (osteogenic).
No statistical significances were observed (adipogenic,
p = 0.31: chondrogenic, p = 0.68, and osteogenic,
p = 0.55). NS: no significant difference.
Table 1.
Flow cytometry analyses of cell surface marker antigens.
Expression of mesenchymal markers (CD29, CD90, CD105),
endothelial markers (CD31, CD146, Flk-1), a stem cell
marker (CD34), and a hematopoietic marker (CD45) of
ASCs cultured with DMEM or EGM-2 was quantitatively
examined at passages 0?3.
|

