|
Abstract
Background: Although an aggressive use of tretinoin along
with hydroquinone enables an efficient treatment of hyperpigmented
skin lesions, irritant dermatitis remains to be solved.
Objective: To determine the effectiveness of 10 % all-trans
retinol (ROL) gel for improvement of skin hyperpigmentation.
Methods: 10 % ROL gel was used instead of 0.1 % tretinoin
gel in our two-phased bleaching protocol (bleaching and healing
phases). 5% hydroquinone and 7% lactic acid ointment was
used along with ROL gel in the bleaching phase (2-6 weeks).
5% hydroquinone and 7% ascorbic acid ointment was used alone
during the healing phase (4-6 weeks). Twenty-one Japanese
patients with hyperpigmented lesions on the face were enrolled
in this study, and 18 patients followed up for more than
10 weeks were analyzed.
Results: Improvement of pigmentation was seen in 16 of 18
patients after an average treatment period of 11.3 weeks,
and in 6 patients pigmentation was almost eliminated after
treatment. Erythema and scaling were seen, however, during
the bleaching phase as well as the bleaching treatment with
tretinoin gel.
Conclusion: ROL can improve skin hyperpigmentation to a similar
extent to tretinoin when used at high concentration, whereas
it induces irritant dermatitis as well.
Introduction
Tretinoin and hydroquinone have been used successfully for
treatment of skin hyperpigmentation since Kligman and Willis
[1] introduced their bleaching formula. Several modified
protocols have been reported [2], and the authors also proposed
an aggressive bleaching therapy, in which tretinoin and hydroquinone
were used separately and corticosteroids were not used [3-5].
In our facility, more than 8,500 cases with various kinds
of hyperpigmented lesions have been successfully treated
with our protocol and its modifications since 1995. Virtually
the only adverse side effect of the strong bleaching treatment
is irritant dermatitis. Further investigations and clinical
trials would be worthwhile, in order to reduce the displeasing
side effects without losing bleaching ability.
All-trans retinol (ROL) and all-trans retinal (RAL) have
been considered to be less irritating than tretinoin if ROL
or RAL was applied at relatively low (0.1-1.6 %) concentration
[6, 7]. However, since binding affinity to specific nuclear
receptors (retinoic acid receptors; RARs) of ROL or RAL is
far less than that of tretinoin, beneficial effects are also
expected to be very moderate compared to tretinoin. Our previous
study revealed that HB-EGF mRNA, which play a critical role
in epidermal growth after retinoids treatment [8, 9], was
upregulated by ROL or RAL to a similar extent as tretinoin,
but only when ROL or RAL was used at 40-100 times higher
concentration than tretinoin [10]. We assumed that HB-EGF
mRNA is one of the important indexes of retinoids at least
when they are used for bleaching.
Based on the data mentioned above, we prepared 10% ROL aqueous
gel, because ROL gel of 100 times higher concentration than
tretinoin can be expected to show similar clinical effects
to tretinoin gel (0.1%). The ROL gel was clinically used
for depigmenting in order to estimate its bleaching potential
and the extent of adverse side effects.
Patients and Methods
Preparation of Ointments: ROL aqueous gel (10%) was originally
prepared and packed into small stainless tubes (5 g each)
at the Department of Pharmacy, University of Tokyo Graduate
School of Medicine. The precise regimen of ROL aqueous gel
for 100 g was as follows: all-trans retinol (Sigma Chemical,
St. Louis, MO) 10 g, Carbopol 940 (Goodrich Chemical, Hounlow,
UK) 1 g, polyoxyethylene oleyl ether (Kao, Tokyo, Japan)
2.5 g, methyl p-hydroxybenzoate (Wako Pure Chemical Industries,
Osaka, Japan) 0.026 g, propyl p-hydroxybenzoate (Wako Pure
Chemical Industries, Osaka, Japan) 0.014 g, 10% sodium hydroxide
aqueous solution 0.6 ml, purified water 86 g. An ointment
including 5% hydroquinone and 7% lactic acid (HQ-LA ointment),
and an ointment including 5% hydroquinone and 7% ascorbic
acid (HQ-AA ointment) were also prepared. Plastibase (petrolatum
polyethylene ointment base, Taisho Pharmacology, Osaka, Japan)
was used as the ointment base of the HQ-LA ointment, while
the hydrophilic ointment was used for the HQ-AA ointments.
Because ROL gel, HQ-LA, and HQ-AA ointments (especially ROL
gel) are pharmacologically unstable, fresh ointments were
prepared at least once a month and stored in a dark and cool
(4oC) place.
Evaluations of results:
Photographs were taken for every patient at baseline and
after treatment with a high-resolution digital camera (Canon
EOS-D30). The percentage of pigmentary clearance was evaluated
via the photographs by two experienced plastic surgeons that
did not perform this treatment. The mean data of the pigmentary
clearance of each patient were classified into 4 categories: "excellent" (90%
or more clearance), "good" (60% to less than 90%
clearance), "fair" (30% to less than 60% clearance),
and "poor" (less than 30% clearance).
Patients: Each ointment was topically applied under signed
informed consent in 21 Japanese women with hyperpigmented
skin lesions on the face, and 18 of them followed up for
more than 10 weeks were analyzed in this study. The other
3 patients stopped treatment because of irritant dermatitis
induced by ROL gel and/or hydroquinone. The age of patients
varied from 20 to 67 years old (age=43.2±7.0; mean S.D.).
Thirteen patients had solar lentigines, 5 had melasma, and
4 had ephelides; 4 patients had two kinds of pigmented lesions.
Treatment protocol: Our bleaching protocol is composed of
two phases, a bleaching phase and a healing phase. In the
bleaching phase, the pigmentation is aggressively treated,
and transient adverse skin effects such as erythema and irritation
are usually observed. Once satisfactory improvement is obtained,
the healing phase is started in order to reduce the erythema
and inflammation, taking care not to induce new postinflammatory
hyperpigmentation.
1) bleaching phase: 10 % ROL gel and HQ-LA ointment were
applied twice a day. ROL gel was carefully applied only on
pigmented areas using a small cotton-tip applicator, and
subsequently HQ-LA ointment was widely applied with the fingers
beyond the pigmented area (e.g. all over the face). Patients
were requested to visit our hospital at 1, 2, 4, 6 and 8
weeks after starting this treatment, and every 2 weeks afterwards.
In most cases, it took 2 to 6 weeks to finish this phase.
2) healing phase: After sufficient improvement of pigmentation
was obtained, the application of ROL gel and HQ-LA ointment
was discontinued, and application of HQ-AA ointment all over
the face was started. HQ-AA ointment was used until the erythema
was almost eliminated, and it took 4-6 weeks to complete
this phase. Topical corticosteroids were not employed either
in the bleaching or healing phase.
Results
The average treatment period of 18 patients was 11.3 weeks,
ranged from 8 to 16 weeks. Erythema and scaling started to
be seen during the first week in most cases. Comparing with
thousands of our cases treated with 0.1 % tretinoin gel (our
preparation regimen is available in ref. #4), we thought
that 10 % ROL gel induced slightly less degrees of erythema
and higher degrees of scaling in the first two weeks, although
statistical analysis was not performed. In addition, tolerance
to the active reagent seemed to be acquired earlier in the
ROL treatment than with tretinoin. In some patients who achieved
tolerance as early as 2 weeks, further improvement was unlikely,
so the results were categorized as "poor" and "fair".
The clinical results were summarized in Table 1. Six patients
(33.3 %) were evaluated as "excellent", 7 cases
(38.9 %) as "good", 3 cases (16.7 %) as "fair",
and 2 cases (11.1 %) as "poor". Some improvement
was seen in 16 of 18 patients (88.9 %). Most of "fair" and "poor" cases
had very mild or no skin reactions such as scaling and erythema
during the bleaching phase. The representative 4 cases are
shown in Figs. 1-4.
Discussion
In bleaching treatment with tretinoin and hydroquinone, we
believe that the role of tretinoin is to wash out of melanin
granules out of epidermis, while that of hydroquinone is
to strongly suppress new melanin production [10, 11]. It
is suggested that tretinoin shows a depigmenting effect by:
1) accelerating epidermal turnover (differentiation of keratinocytes),
and also 2) promoting epidermal growth (proliferation of
keratinocytes); the latter of the two effects was found to
be mediated by heparin-binding EGF-like growth factor (HB-EGF)
secreted by suprabasal keratinocytes [8, 9, 12]. Thus, the
mechanism of this bleaching therapy is very simple; accelerate
the output, and suppress the input, but appears to be only
effective for epidermal pigmentation. Indeed, we confirmed
histologically that accumulated melanin granules around the
basal layer were cleared up after treating with tretinoin,
but the melanin deposits in the dermis appeared not to change
in acquired bilateral nevus of Ota-like macules, which has
both epidermal and dermal pigmentation (in preparation).
The authors recently reported that ROL and RAL can induce
markedly HB-EGF mRNA upregulation at pharmacological concentration
(0.01-1 mM) in cultured human keratinocytes [10]. However,
in cases of ROL and RAL, 40-100 times higher concentration
was required in order to upregulate HB-EGF mRNA to a similar
extent to tretinoin [307]. In the present study, we prepared
and used 10 % ROL gel, which is 100 times higher concentration
that 0.1% tretinoin gel, and our subjective opinion is that
10% ROL gel is as effective as 0.1% tretinoin gel in improving
skin hyperpigmentation based on our experience with thousands
of patients treated with 0.1% tretinoin gel.
ROL and RAL are considered to work after conversion to tretinoin.
The binding affinities of Rol and Ral to RARs are quite low
[13], so that their biological activity should result from
their oxidative transformation into tretinoin by epidermal
keratinocytes. This conversion to tretinoin after topical
application of ROL and RAL was revealed to occur in a study
using skin organ culture [14]. However, there have been some
reports suggesting the existence of other pathways than that
mediated by nuclear receptors [15]. ROL is known to show
much less side effects such as irritation than tretinoin,
at least when used at similar concentration, thus has been
thought to be more tolerable, and may be of great value in
clinical use. However, the present study revealed that even
ROL can induce irritant contact dermatitis to a similar extent
as tretinoin when used at 100 times higher concentration,
although it also showed beneficial effects similar to tretinoin.
It remains unknown what percentages of ROL in the prepared
ROL gel was absorbed into the skin. Unlike the 0.1 % tretinoin
gel we use in which more than 96 % of the gel is composed
of water, the 10 % ROL gel contains 10 % ROL, while water
formed only 86 % of it. The permeability of ROL in the 10
% ROL gel may be much less than that of tretinoin in the
0.1% tretinoin gel. Therefore, 5 % ROL gel which should contain
more water may show similar depigmenting effects to the 10
% ROL gel.
In this study, we showed that ROL can play a depigmenting
role as well as tretinoin. However, the cost performance
of the ROL gel is very poor, compared to the tretinoin gel,
as far as our preparations are concerned. Purified ROL is
commercially available, but 1 gram of it costs almost the
same as 1 gram of tretinoin. Since 100 times amount of the
working ingredient was required to prepare the ROL gel, it
costs 100 times more. In light of this, it seems to be impractical
to clinically use the 10 % ROL gel we prepared.
Conclusions
The present clinical trial revealed that 10 % ROL gel clinically
show a considerable bleaching effect, which seems to be as
good as that of 0.1 % tretinoin gel, but the ROL gel also
induce adverse side effects, such as irritant dermatitis,
to a similar degrees as tretinoin gel.
References
1. Kligman AM, Willis
I. A new formula for depigmenting human skin.
Arch Dermatol 1975; 111: 40-8.
2. Gano SE, Garcia RL. Topical tretinoin, hydroquinone, and
betamethasone valerate in the therapy of melasma. Cutis 1979;
23: 239-41.
3. Yoshimura K, Harii K, Shibuya F, Aoyama T, Iga T. A new
bleaching protocol for hyperpigmented skin lesions with a
high concentration of all-trans retinoic acid aqueous gel.
Aesthetic Plast Surg 1999; 23: 285-91.
4. Yoshimura K, Harii K, Aoyama T, Iga T. Experience of a
strong bleaching treatment for skin hyperpigmentation in
Orientals. Plast Reconstr Surg 2000; 105: 1097-108.
5. Yoshimura K, Harii K, Masuda Y, Takahashi M, Aoyama T,
Iga T. Usefulness of a narrow-band reflectance spectrophotometer
in evaluating effects of depigmenting treatment. Aesthetic
Plast Surg 2001; 25: 129-33.
6. Kang S, Duell EA, Fisher GJ, et al. Application of retinol
to human skin in vivo induces epidermal hyperplasia and cellular
retinoid binding proteins characteristic of retinoic acid
but without measurable retinoic acid levels or irritation.
J Invest Dermatol 1995 ;105: 549-56.
7. Fluhr JW, Vienne MP, Lauze C, Dupuy P, Gehring W, Gloor
M. Tolerance profile of retinol, retinaldehyde and retinoic
acid under maximized and long-term clinical conditions. Dermatology
1999; 199(S1): 57-60.
8. Xiao JH, Feng X, Di W, et al. Identification of heparin-binding
EGF-like growth factor as a target in intercellular regulation
of epidermal basal cell growth by suprabasal retinoic acid
receptors. EMBO J 1999; 18: 1539-48.
9. Varani J, Zeigler M, Dame MK, et al. Heparin-binding epidermal-growth-factor-like
growth factor activation of keratinocyte ErbB receptors Mediates
epidermal hyperplasia, a prominent side-effect of retinoid
therapy. J Invest Dermatol. 2001; 117: 1335-41.
10. Yoshimura K, Uchida G, Okazaki M, Kitano Y, Harii K.
Differential expression of heparin-binding EGF-like growth
factor (HB-EGF) mRNA in normal human keratinocytes induced
by a variety of natural and synthetic retinoids. Exp Dermatol,
in press.
11. Yoshimura K, Tsukamoto K, Okazaki M, et al. Effects of
all-trans retinoic acid on melanogenesis in pigmented skin
equivalents and monolayer culture of melanocytes. J Dermatol
Sci 2001; 27(S1): 68-75.
12. Stoll SW, Elder JT. Retinoid regulation of heparin-binding
EGF-like growth factor gene expression in human keratinocytes
and skin. Exp Dermatol 1998; 7: 391-7.
13. Crettaz M, Baron A, Siegenthaler G, Hunziker W. Ligand
specificities of recombinant retinoic acid receptors RA alpha
and RAR beta. Biochem J 1990: 272: 391-7.
14. Bailly J, Crettaz M, Schifflers MH, Marty JP. In vitro
metabolism by human skin and fibroblasts of retinol, retinal
and retinoic acid. Exp Dermatol 1998; 7: 27-34.
15. Saurat JH, Sorg O, Didierjean L. New concepts for delivery
of topical retinoid activity to human skin. In: Nau H, Blaner
W S, ed. Retinoids. Berlin: Springer, 1999: 521-38.
Figure Legends
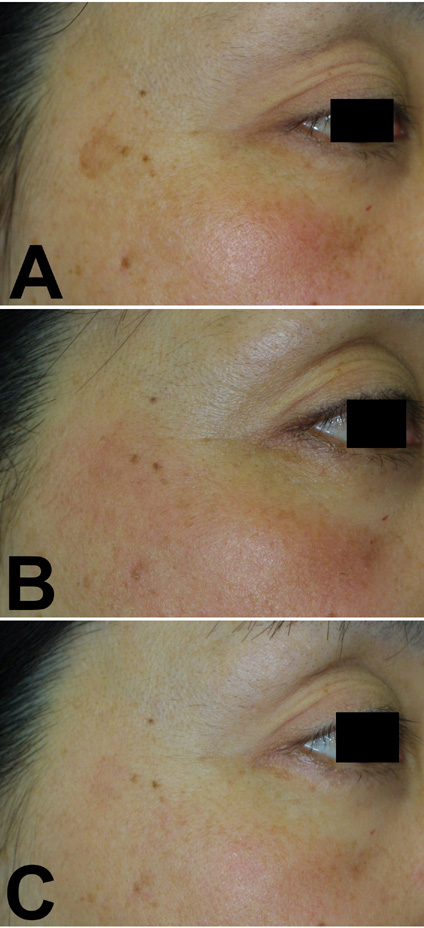
Fig.1. Case 1. A
46-year-old woman with a solar lentigine and
melasma on her cheek underwent the treatment
(A: before treatment); 10 % ROL gel was used
for 4 weeks together with HQ-LA ointment (B:
at 4 weeks; after bleaching treatment), followed
by application of HQ-AA ointment alone for 4
weeks. Both the solar lentigine and melasma almost
disappeared after 8 weeks of treatment (C: at
8 weeks).
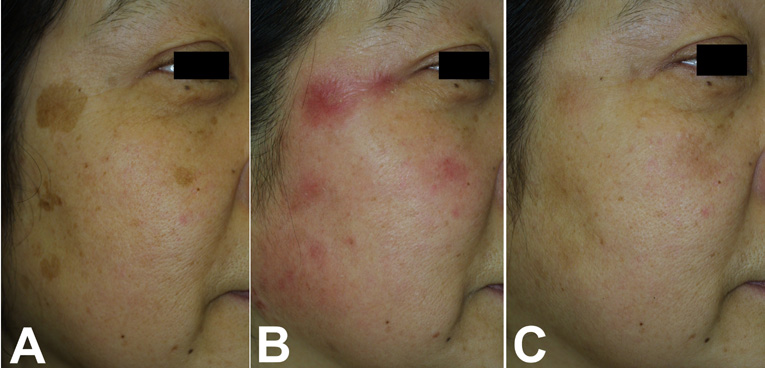
Fig.2. Case 2. A
54-year-old woman with several solar lentigines
on her right cheek underwent the treatment (A:
before treatment); 10 % ROL gel was used for
2 weeks together with HQ-LA ointment, followed
by application of HQ-AA ointment alone for 6
weeks. Significant erythema and irritation on
the treated area were observed 1-3 weeks after
starting the treatment (B: at 2 weeks; after
bleaching phase). The erythema almost disappeared
after 6 weeks' healing phase, although slight
postinflammatory hyperpigmentation was left on
the treated areas (C: at 8 weeks).
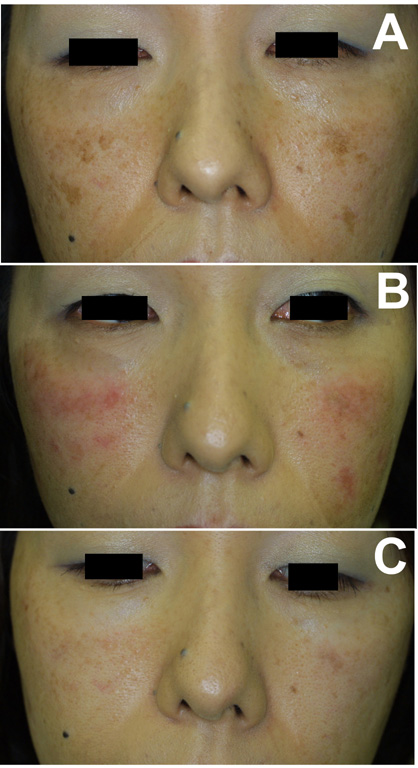
Fig.3. Case 3. A
32-year-old woman with melasma and some solar
lentigines underwent the treatment (A: before
treatment). 10 % ROL gel was used for 4 week
combined with HQ-LA ointment, followed by single
application of HQ-AA ointment for 4 weeks. Irritant
dermatitis was seen on the treated areas throughout
the bleaching phase, but pigmentation was improved
significantly by day 28 (B: at 4 weeks; after
bleaching phase). After 4 weeks of healing phase,
erythema disappeared without leaving postinflammatory
hyperpigmentation (C: at 8 weeks).
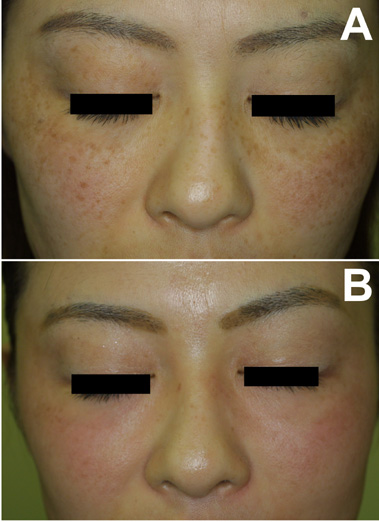
Fig.4. Case 4. A
34-year-old woman with ephelides underwent the
treatment (A: before treatment). Bleaching was
performed with 10 % ROL gel and HQ-LA ointment
for 4 weeks, thereafter HQ-AA ointment alone
was used for 6 weeks. The pigmented spots were
almost eliminated and mild rosy glow was still
seen at 10 weeks (B: at 10 weeks).
|

